444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The single-use medical device reprocessing market is witnessing significant growth due to the increasing focus on cost containment in healthcare facilities and the growing demand for sustainable healthcare practices. Single-use medical device reprocessing refers to the process of cleaning, disinfecting, and sterilizing single-use devices (SUDs) to make them safe for reuse. Reprocessing helps in reducing medical waste and lowers the overall healthcare expenditure. This market is driven by the rising prevalence of chronic diseases, the need to reduce medical waste, and the growing awareness about environmental sustainability.
Meaning
Single-use medical device reprocessing involves the cleaning, disinfection, and sterilization of single-use devices that are typically designed for one-time use. These devices include items such as catheters, surgical blades, electrosurgical instruments, and endoscopes. Reprocessing these devices involves a thorough cleaning process, followed by disinfection and sterilization, to ensure that they can be safely reused without compromising patient safety. The reprocessed devices undergo rigorous testing and quality checks to meet regulatory standards before they are reintroduced into the healthcare setting.
Executive Summary
The single-use medical device reprocessing market is experiencing steady growth, driven by the increasing demand for cost-effective healthcare solutions and the rising emphasis on sustainable practices. Reprocessing single-use devices not only helps in reducing medical waste but also contributes to significant cost savings for healthcare facilities. The market is characterized by the presence of several established players and intense competition. Regulatory frameworks and guidelines play a crucial role in ensuring the safety and efficacy of reprocessed devices. The market is expected to witness continued growth in the coming years, driven by technological advancements, increasing adoption by healthcare providers, and rising environmental concerns.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
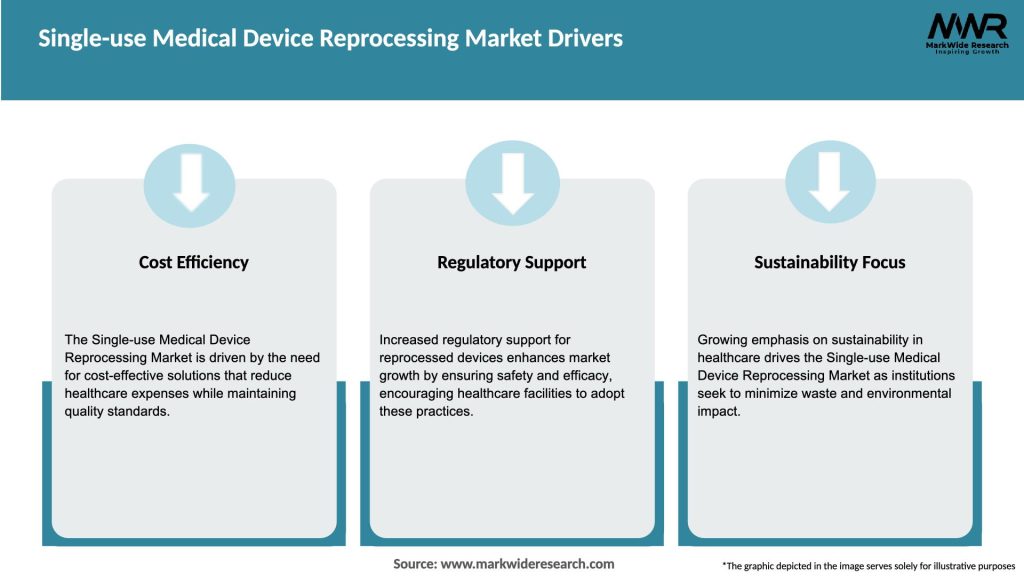
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The single-use medical device reprocessing market is influenced by various dynamic factors, including technological advancements, regulatory landscape, healthcare expenditure, and environmental concerns. The market dynamics play a crucial role in shaping the growth trajectory of the market. Technological advancements in reprocessing techniques and equipment are enabling more efficient and effective cleaning and sterilization, thereby increasing the acceptance of reprocessed devices. Additionally, regulatory support and guidelines ensure the safety and efficacy of reprocessed devices, fostering market growth. The shift towards value-based care and cost containment strategies also drives the adoption of reprocessed devices. However, challenges such as stringent regulations, limited acceptance by some healthcare professionals, and potential risks associated with reprocessed devices can hamper market growth.
Regional Analysis
The single-use medical device reprocessing market can be analyzed on a regional basis, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America currently holds a significant share of the market due to the well-established healthcare infrastructure, favorable reimbursement policies, and increased adoption of reprocessed devices. Europe is also a prominent market, driven by stringent regulatory requirements and the presence of major market players. The Asia Pacific region is witnessing rapid growth due to the increasing healthcare expenditure, rising awareness about sustainable practices, and the growing burden of chronic diseases. Latin America, the Middle East, and Africa offer untapped opportunities for market players to expand their presence in these regions.
Competitive Landscape
Leading Companies in the Single-use Medical Device Reprocessing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
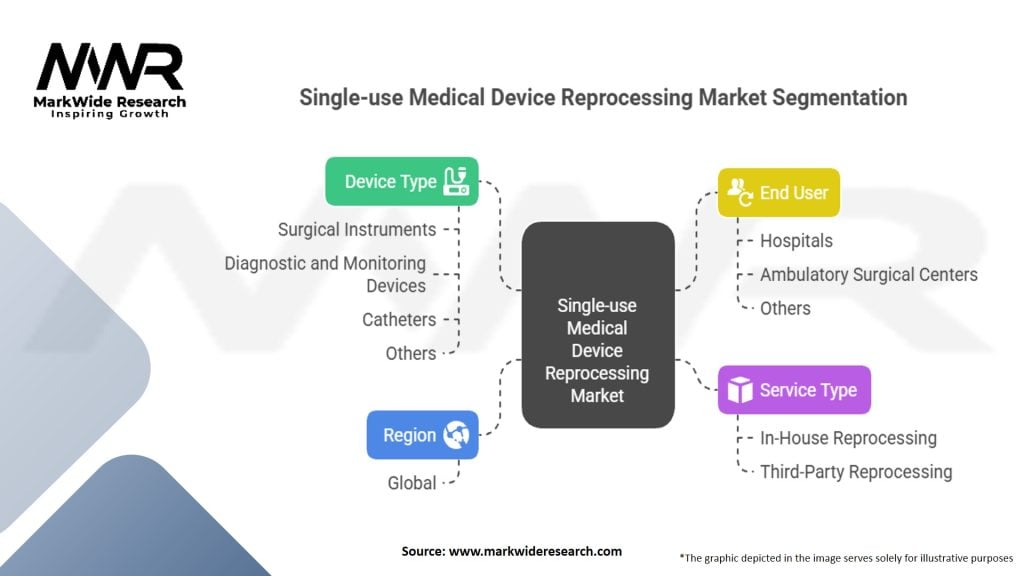
Segmentation
The single-use medical device reprocessing market can be segmented based on the following factors:
Segmenting the market based on these factors helps in understanding the specific needs and demands of different customer segments, enabling market players to tailor their offerings accordingly.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic had a significant impact on the single-use medical device reprocessing market. The pandemic led to an increased demand for medical devices, including single-use devices, resulting in shortages and escalating costs. Reprocessing single-use devices became even more crucial during this period as it helped address the supply chain challenges and reduce the burden on healthcare facilities. However, there were concerns about the potential transmission of the virus through reprocessed devices, leading to stricter guidelines and protocols for reprocessing. The pandemic underscored the need for sustainable healthcare practices and reinforced the importance of reprocessing in reducing medical waste and ensuring the availability of critical medical devices.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the single-use medical device reprocessing market looks promising, with steady growth anticipated. The increasing emphasis on cost containment, sustainability, and the adoption of value-based care models will drive the demand for reprocessed devices. Technological advancements will continue to enhance the efficiency and safety of reprocessing processes, leading to increased acceptance by healthcare professionals and facilities. Regulatory frameworks and guidelines will play a crucial role in shaping the market, ensuring the safety and efficacy of reprocessed devices. The market is expected to witness continued expansion into emerging regions, as well as strategic collaborations and partnerships to foster innovation and address market challenges.
Conclusion
The single-use medical device reprocessing market is experiencing growth due to cost containment initiatives, sustainability concerns, and increasing awareness about the benefits of reprocessed devices. Reprocessing single-use devices provides cost savings, promotes environmental sustainability, and ensures a continuous supply of critical medical devices. The market is dynamic, driven by technological advancements, regulatory landscape, and the shift towards value-based care. While challenges exist, such as stringent regulations and limited acceptance, opportunities for market expansion through emerging markets, collaborations, and innovation are abundant. The future outlook for the market is positive, with continued growth expected as healthcare facilities and professionals recognize the value of single-use medical device reprocessing in improving patient care and reducing healthcare costs.
What is single-use medical device reprocessing?
Single-use medical device reprocessing refers to the practice of cleaning, sterilizing, and reusing medical devices that are typically designed for one-time use. This process aims to reduce medical waste and lower healthcare costs while maintaining safety and efficacy standards.
What are the key companies in the single-use medical device reprocessing market?
Key companies in the single-use medical device reprocessing market include Stryker Corporation, Medline Industries, and ReNu Medical, among others.
What are the main drivers of growth in the single-use medical device reprocessing market?
The growth of the single-use medical device reprocessing market is driven by increasing healthcare costs, a rising focus on sustainability, and the need for efficient resource management in hospitals and clinics.
What challenges does the single-use medical device reprocessing market face?
Challenges in the single-use medical device reprocessing market include regulatory hurdles, concerns about the safety and efficacy of reprocessed devices, and the need for advanced technology to ensure proper sterilization.
What opportunities exist in the single-use medical device reprocessing market?
Opportunities in the single-use medical device reprocessing market include the development of innovative reprocessing technologies, partnerships with healthcare facilities, and expanding into emerging markets where healthcare infrastructure is growing.
What trends are shaping the single-use medical device reprocessing market?
Trends in the single-use medical device reprocessing market include increasing adoption of circular economy principles, advancements in sterilization technologies, and a growing emphasis on environmental sustainability in healthcare practices.
Single-use Medical Device Reprocessing Market
| Segmentation Details | Description |
|---|---|
| Device Type | Surgical Instruments, Diagnostic and Monitoring Devices, Catheters, Others |
| Service Type | In-House Reprocessing, Third-Party Reprocessing |
| End User | Hospitals, Ambulatory Surgical Centers, Others |
| Region | Global |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Single-use Medical Device Reprocessing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at