444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The viral vectors gene therapy market is at the forefront of biomedical innovation, offering promising therapeutic solutions for a wide range of genetic disorders and chronic diseases. Viral vectors serve as vehicles for delivering therapeutic genes into target cells, enabling precise and targeted genetic modifications to correct or replace defective genes, restore normal cellular function, and treat debilitating medical conditions. This market holds immense potential for revolutionizing healthcare and addressing unmet medical needs through the development of safe, effective, and personalized gene-based therapies.
Meaning:
Viral vectors are genetically engineered viruses that have been modified to carry therapeutic genes into specific cells or tissues of the body. These vectors are designed to deliver the therapeutic payload safely and efficiently, allowing for the expression of therapeutic proteins, regulation of gene expression, or correction of genetic defects within the host cells. Viral vectors serve as indispensable tools in gene therapy research and clinical applications, offering precise targeting, high transduction efficiency, and potential long-term therapeutic benefits for patients with genetic disorders, cancer, and other diseases.
Executive Summary:
The viral vectors gene therapy market is witnessing rapid growth and innovation, driven by advances in gene editing technologies, improved vector design, and expanding therapeutic indications. Key market players are investing heavily in research and development to enhance vector safety, efficacy, and delivery, while regulatory agencies are providing streamlined pathways for accelerated approval of gene therapies. Despite challenges such as manufacturing scalability, immunogenicity, and long-term safety concerns, the market shows tremendous promise for transforming the treatment landscape and improving patient outcomes across a spectrum of genetic and acquired diseases.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The viral vectors gene therapy market is characterized by dynamic growth and evolution, driven by technological innovation, scientific advancements, regulatory developments, and market forces. Key dynamics shaping the market include:
Regional Analysis:
The viral vectors gene therapy market exhibits regional variations in market size, growth potential, regulatory frameworks, and healthcare infrastructure. Key regions driving market growth and innovation include:
Competitive Landscape:
Leading Companies in Viral Vectors Gene Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation:
The viral vectors gene therapy market can be segmented based on various factors, including vector type, therapeutic indication, target tissue, and geographical region:
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The COVID-19 pandemic has had both positive and negative impacts on the viral vectors gene therapy market:
Key Industry Developments
Analyst Suggestions
Future Outlook:
The viral vectors gene therapy market is poised for significant growth and innovation in the coming years, driven by advances in gene editing technologies, expanding therapeutic applications, and increasing investment in research, manufacturing, and commercialization. Key trends shaping the future outlook of the market include:
Overall, the viral vectors gene therapy market holds immense promise for transforming the treatment landscape and improving patient outcomes across a wide range of genetic disorders, chronic diseases, and unmet medical needs, driving innovation, investment, and global health impact in the years to come.
Conclusion:
The viral vectors gene therapy market stands at the forefront of medical innovation, offering transformative treatments for a myriad of genetic disorders, chronic diseases, and oncological conditions. As precision medicine continues to evolve, viral vectors gene therapy holds the potential to revolutionize patient care, providing personalized, targeted therapies tailored to individual genetic profiles and disease characteristics.
Despite challenges posed by the COVID-19 pandemic, the market has demonstrated resilience and continued growth, driven by increasing investment in research and development, expanding therapeutic applications, and rising demand for advanced gene-based treatments. Regulatory agencies have shown flexibility in response to the pandemic, expediting review processes for COVID-19-related therapies while maintaining rigorous standards for safety and efficacy.
Looking ahead, the future outlook for the viral vectors gene therapy market is promising, with opportunities for innovation, collaboration, and market expansion. Advances in gene editing technologies, convergence of therapeutic modalities, and regulatory evolution will shape the landscape of gene therapy, unlocking new possibilities for treating previously untreatable diseases and improving patient outcomes worldwide.
In conclusion, the viral vectors gene therapy market represents a paradigm shift in healthcare, offering hope for patients and families affected by genetic disorders and chronic diseases. By harnessing the power of genetic engineering, precision medicine, and collaborative partnerships, the viral vectors gene therapy market is poised to transform the treatment landscape and pave the way for a healthier, more resilient future.
What is Viral Vectors Gene Therapy?
Viral Vectors Gene Therapy refers to a technique that uses modified viruses to deliver genetic material into cells, aiming to treat or prevent diseases by correcting defective genes. This approach is widely used in the treatment of genetic disorders, cancers, and infectious diseases.
What are the key companies in the Viral Vectors Gene Therapy Market?
Key companies in the Viral Vectors Gene Therapy Market include Novartis, Gilead Sciences, and Spark Therapeutics, which are actively involved in developing innovative therapies using viral vectors. These companies focus on various applications, including rare genetic disorders and oncology treatments, among others.
What are the drivers of growth in the Viral Vectors Gene Therapy Market?
The growth of the Viral Vectors Gene Therapy Market is driven by increasing investments in gene therapy research, advancements in viral vector technology, and a rising prevalence of genetic disorders. Additionally, the growing acceptance of personalized medicine is contributing to market expansion.
What challenges does the Viral Vectors Gene Therapy Market face?
The Viral Vectors Gene Therapy Market faces challenges such as high development costs, regulatory hurdles, and potential safety concerns associated with viral vector use. These factors can hinder the speed of bringing new therapies to market.
What opportunities exist in the Viral Vectors Gene Therapy Market?
Opportunities in the Viral Vectors Gene Therapy Market include the potential for developing therapies for previously untreatable conditions and the expansion of applications in oncology and rare diseases. Collaborations between biotech firms and research institutions are also fostering innovation.
What trends are shaping the Viral Vectors Gene Therapy Market?
Trends in the Viral Vectors Gene Therapy Market include the increasing use of adeno-associated viruses (AAV) for gene delivery and the rise of CRISPR technology for gene editing. Additionally, there is a growing focus on improving the efficiency and safety of viral vectors.
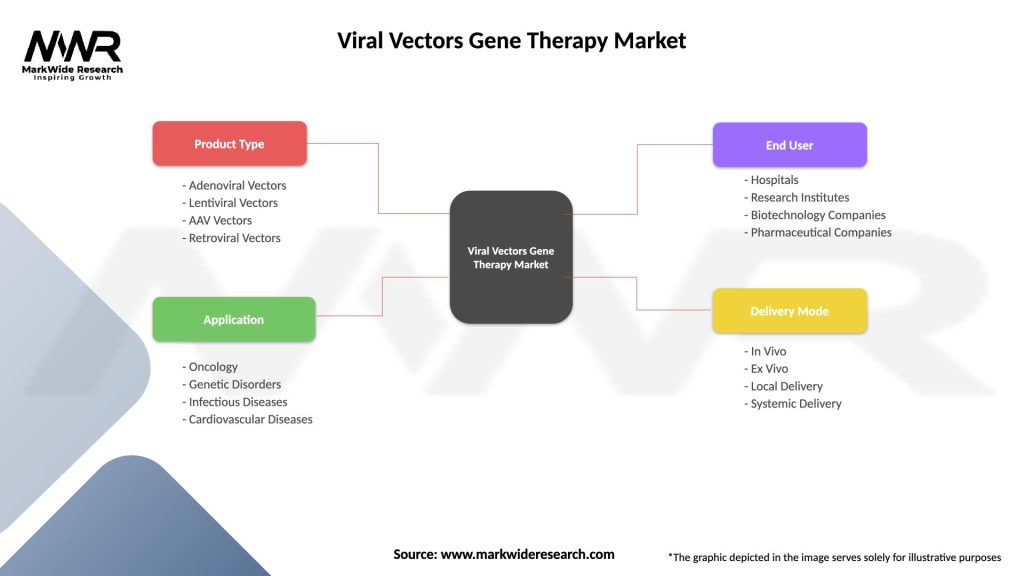
Viral Vectors Gene Therapy Market
| Segmentation Details | Description |
|---|---|
| Product Type | Adenoviral Vectors, Lentiviral Vectors, AAV Vectors, Retroviral Vectors |
| Application | Oncology, Genetic Disorders, Infectious Diseases, Cardiovascular Diseases |
| End User | Hospitals, Research Institutes, Biotechnology Companies, Pharmaceutical Companies |
| Delivery Mode | In Vivo, Ex Vivo, Local Delivery, Systemic Delivery |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Viral Vectors Gene Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at