444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The Vaporized Hydrogen Peroxide (VHP) Sterilization Systems for Medical Devices Market is a pivotal sector within the healthcare industry, providing advanced sterilization solutions for medical devices, instruments, and equipment in healthcare facilities, pharmaceutical manufacturing, and research laboratories. Vaporized hydrogen peroxide sterilization systems offer rapid, effective, and environmentally friendly sterilization methods, ensuring patient safety, infection control, and regulatory compliance in healthcare settings.
Meaning:
Vaporized hydrogen peroxide sterilization systems utilize hydrogen peroxide vapor to decontaminate and sterilize medical devices, surgical instruments, and laboratory equipment by disrupting microbial cell membranes, denaturing proteins, and oxidizing cellular components, resulting in microbial inactivation and elimination of pathogenic microorganisms, including bacteria, viruses, and spores. These sterilization systems offer advantages such as low temperature operation, material compatibility, and cycle time efficiency, making them ideal for heat-sensitive medical devices and complex instrument sets.
Executive Summary:
The Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market is experiencing robust growth, driven by factors such as increasing emphasis on infection prevention, rising demand for sterilization technologies, and technological advancements in hydrogen peroxide vaporization systems. Key trends include the adoption of hydrogen peroxide plasma sterilization, integration of IoT-enabled features, and expansion of sterilization service offerings in healthcare facilities and contract sterilization facilities.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market operates within a dynamic ecosystem shaped by factors such as technological innovation, market demand, and regulatory landscape dynamics. Market players must navigate these dynamics by leveraging strategic partnerships, customer insights, and continuous innovation to develop differentiated products, expand market presence, and sustain competitive advantage in the evolving sterilization market landscape.
Regional Analysis:
Competitive Landscape:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
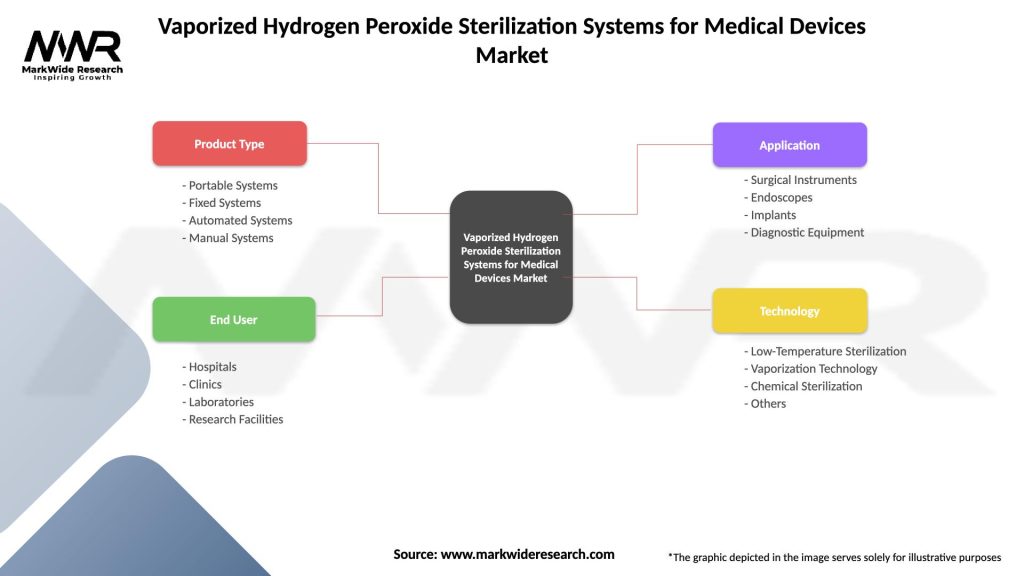
Segmentation:
The market can be segmented based on factors such as product type, end-user, application, and geographical region. Common segmentation categories include:
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has underscored the critical role of vaporized hydrogen peroxide sterilization systems in infection prevention, pandemic response, and healthcare resilience, as healthcare facilities worldwide prioritize sterilization capacity, surge preparedness, and patient safety initiatives to combat the pandemic. Vaporized hydrogen peroxide sterilization systems play a vital role in decontaminating personal protective equipment (PPE), medical devices, and critical supplies to mitigate the spread of Covid-19, protect frontline healthcare workers, and ensure healthcare system readiness in pandemic response efforts.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The future outlook for the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market is optimistic, driven by factors such as technological innovation, market demand, and regulatory landscape dynamics in healthcare, pharmaceutical manufacturing, and research laboratory sectors. Market players must continue to innovate, collaborate, and adapt to evolving market trends, customer needs, and regulatory requirements to maintain competitiveness, foster innovation, and sustain growth in the dynamic sterilization market landscape.
Conclusion:
In conclusion, the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market represents a critical segment of the healthcare industry, providing essential sterilization solutions for medical devices, surgical instruments, and laboratory equipment in healthcare facilities, pharmaceutical manufacturing, and research laboratories. Despite challenges such as capital investment requirements, validation complexities, and competitive alternatives, vaporized hydrogen peroxide sterilization systems offer significant benefits, including high sterilization efficacy, material compatibility, and environmental sustainability, making them indispensable tools for infection prevention, patient safety, and regulatory compliance in healthcare delivery. By leveraging technological innovation, regulatory compliance, and collaborative partnerships, industry stakeholders can contribute to the advancement of vaporized hydrogen peroxide sterilization systems, drive market growth, and realize the full potential of sterilization technologies in improving healthcare outcomes and ensuring public health and safety worldwide.
What is Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices?
Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices are advanced sterilization technologies that utilize vaporized hydrogen peroxide to effectively eliminate microorganisms on medical instruments and devices. This method is particularly valued for its efficacy in sterilizing heat-sensitive items and its compatibility with various materials used in medical devices.
What are the key players in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market?
Key players in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market include companies like Steris Corporation, Getinge AB, and Advanced Sterilization Products. These companies are known for their innovative sterilization solutions and play a significant role in the healthcare sector, among others.
What are the growth factors driving the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market?
The growth of the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market is driven by the increasing demand for effective sterilization methods in healthcare settings, the rise in surgical procedures, and the need for infection control. Additionally, advancements in sterilization technology and the growing awareness of patient safety contribute to market expansion.
What challenges does the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market face?
Challenges in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market include the high initial investment costs associated with advanced sterilization equipment and the need for proper training of personnel. Furthermore, regulatory compliance and the potential for material compatibility issues can also pose challenges.
What opportunities exist in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market?
Opportunities in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market include the increasing adoption of minimally invasive surgical techniques and the expansion of healthcare facilities in emerging markets. Additionally, the development of new sterilization technologies and products presents further growth potential.
What trends are shaping the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market?
Trends in the Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market include a growing emphasis on environmentally friendly sterilization methods and the integration of automation in sterilization processes. Moreover, the rising focus on infection prevention and control in healthcare settings is driving innovation in this field.
Vaporized Hydrogen Peroxide Sterilization Systems for Medical Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Portable Systems, Fixed Systems, Automated Systems, Manual Systems |
| End User | Hospitals, Clinics, Laboratories, Research Facilities |
| Application | Surgical Instruments, Endoscopes, Implants, Diagnostic Equipment |
| Technology | Low-Temperature Sterilization, Vaporization Technology, Chemical Sterilization, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at