444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The vaporized hydrogen peroxide (VHP) disinfection equipment market for medical devices is experiencing significant growth, fueled by the increasing emphasis on infection control in healthcare settings, rising demand for sterilization solutions, and advancements in VHP technology. VHP disinfection systems offer rapid, efficient, and environmentally friendly methods for decontaminating medical devices, ensuring patient safety and preventing healthcare-associated infections.
Meaning
Vaporized hydrogen peroxide (VHP) disinfection equipment utilizes gaseous hydrogen peroxide vapor to sterilize medical devices, equipment, and surfaces. VHP is a potent oxidizing agent that penetrates and destroys microbial contaminants, including bacteria, viruses, and spores, without leaving harmful residues or requiring extensive post-processing. VHP disinfection systems employ specialized generators, distribution systems, and monitoring technologies to deliver consistent and validated sterilization cycles.
Executive Summary
The vaporized hydrogen peroxide disinfection equipment market for medical devices is witnessing robust growth, driven by the increasing awareness about infection prevention, regulatory mandates for sterilization standards, and technological advancements in VHP systems. Key players in the market are investing in product development, regulatory compliance, and market expansion initiatives to address the evolving needs of healthcare providers and improve patient safety outcomes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The vaporized hydrogen peroxide disinfection equipment market is characterized by dynamic trends such as the integration of VHP systems with robotics, artificial intelligence, and Internet of Things (IoT) technologies. Market players are focusing on enhancing system performance, user interface design, and data analytics capabilities to optimize sterilization processes, ensure regulatory compliance, and improve workflow efficiency.
Regional Analysis
The market for vaporized hydrogen peroxide disinfection equipment is geographically diverse, with North America leading in terms of market share due to a high prevalence of healthcare-associated infections, stringent sterilization standards, and a well-developed healthcare infrastructure. However, Europe, Asia-Pacific, and Latin America regions are also witnessing significant growth driven by increasing investments in healthcare infrastructure, rising demand for medical device sterilization solutions, and regulatory harmonization efforts.
Competitive Landscape
Leading Companies in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
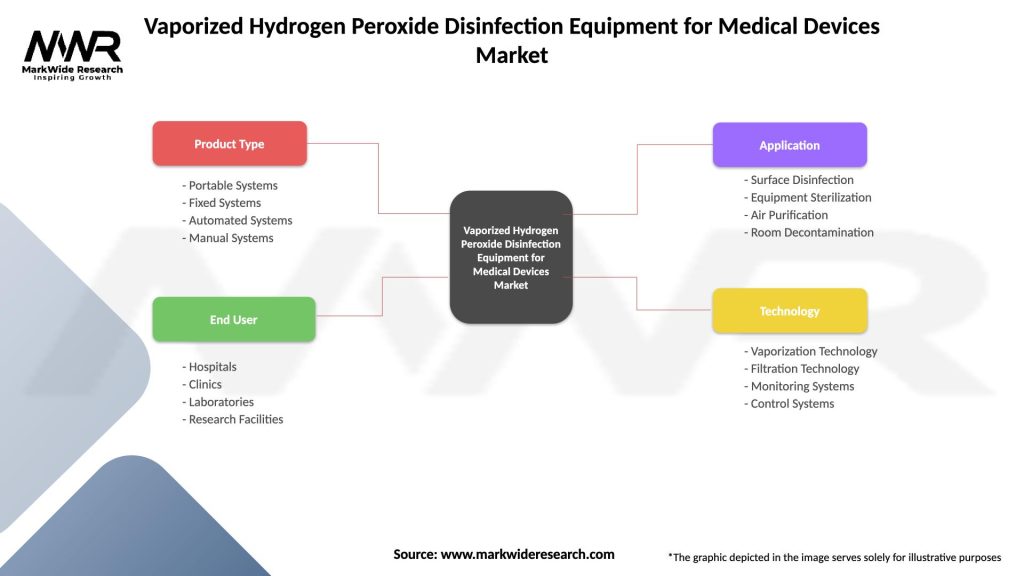
The vaporized hydrogen peroxide disinfection equipment market can be segmented based on product type, application, end-user, and geography. Product types include VHP generators, vaporizers, monitoring systems, and accessories, each designed for specific sterilization requirements and facility configurations. Applications encompass medical device reprocessing, cleanroom decontamination, laboratory sterilization, and pharmaceutical manufacturing. End-users of VHP disinfection equipment include hospitals, clinics, laboratories, pharmaceutical companies, contract sterilization service providers, and research institutions.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the vaporized hydrogen peroxide disinfection equipment market can benefit from:
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has heightened the demand for vaporized hydrogen peroxide disinfection equipment for medical devices, driven by the need for rapid, reliable, and scalable sterilization solutions to mitigate infection risks in healthcare facilities. While the pandemic initially strained supply chains and increased demand volatility, it also underscored the importance of infection control, sterilization validation, and healthcare preparedness. As healthcare systems adapt to the challenges posed by the pandemic, the demand for VHP sterilization technology is expected to remain strong in the post-pandemic era.
Key Industry Developments
Analyst Suggestions
Analysts recommend industry players to focus on innovation, collaboration, and market expansion strategies to capitalize on emerging opportunities and address market challenges in the dynamic vaporized hydrogen peroxide disinfection equipment market. Investing in research and development, regulatory compliance, and customer education is essential for driving market growth and differentiation in the evolving sterilization technology landscape.
Future Outlook
The future outlook for the vaporized hydrogen peroxide disinfection equipment market is optimistic, with sustained growth expected driven by increasing awareness about infection control, rising demand for sterilization solutions, and advancements in VHP technology. Market players are well-positioned to capitalize on the opportunities presented by the growing need for rapid, efficient, and environmentally friendly sterilization methods in healthcare, life sciences, and critical infrastructure sectors.
Conclusion
In conclusion, the vaporized hydrogen peroxide disinfection equipment market for medical devices presents significant opportunities for industry participants and stakeholders seeking to advance infection control, improve patient safety, and enhance healthcare quality. By leveraging innovation, collaboration, and market insights, businesses can develop and commercialize VHP sterilization systems that offer rapid, efficient, and reliable solutions for decontaminating medical devices, equipment, and facilities. With the right strategies and investments, the vaporized hydrogen peroxide disinfection equipment market has the potential to drive transformative changes in infection prevention, healthcare sustainability, and public health worldwide.
What is Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices?
Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices refers to specialized systems that utilize vaporized hydrogen peroxide to effectively sterilize medical instruments and devices. This technology is widely used in healthcare settings to ensure the safety and cleanliness of surgical tools and other equipment.
What are the key players in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market?
Key players in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market include companies like Steris Corporation, Getinge AB, and Advanced Sterilization Products. These companies are known for their innovative sterilization solutions and play a significant role in the healthcare industry, among others.
What are the growth factors driving the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market?
The growth of the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market is driven by increasing healthcare-associated infections, the rising demand for sterilization in hospitals, and advancements in disinfection technologies. Additionally, the growing awareness of infection control practices contributes to market expansion.
What challenges does the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market face?
Challenges in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market include the high initial investment costs and the need for proper training to operate the equipment effectively. Furthermore, regulatory compliance and the availability of alternative disinfection methods can also pose challenges.
What opportunities exist in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market?
Opportunities in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market include the increasing adoption of advanced sterilization technologies and the expansion of healthcare facilities globally. Additionally, the growing focus on sustainable and eco-friendly disinfection methods presents new avenues for growth.
What trends are shaping the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market?
Trends in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market include the integration of automation in disinfection processes and the development of portable disinfection units. There is also a rising interest in combining vaporized hydrogen peroxide with other disinfection technologies to enhance efficacy.
Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Portable Systems, Fixed Systems, Automated Systems, Manual Systems |
| End User | Hospitals, Clinics, Laboratories, Research Facilities |
| Application | Surface Disinfection, Equipment Sterilization, Air Purification, Room Decontamination |
| Technology | Vaporization Technology, Filtration Technology, Monitoring Systems, Control Systems |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Vaporized Hydrogen Peroxide Disinfection Equipment for Medical Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at