444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview:
The US Tardive Dyskinesia (TD) therapeutics market addresses the treatment landscape for a neurological disorder characterized by involuntary, repetitive movements, primarily affecting the face and extremities. TD often occurs as a side effect of long-term use of certain medications, especially antipsychotics. The market focuses on developing therapeutic interventions to manage and alleviate the symptoms of TD, improving the quality of life for affected individuals.
Meaning:
Tardive Dyskinesia is a neurological condition characterized by involuntary, repetitive movements, including facial grimacing, tongue protrusion, and choreiform movements of the extremities. It typically arises as a side effect of prolonged use of dopamine-blocking medications, particularly antipsychotics. The meaning of TD in the context of therapeutics involves developing targeted approaches to mitigate symptoms and enhance patient well-being.
Executive Summary:
The US Tardive Dyskinesia therapeutics market has witnessed notable advancements in recent years, driven by a growing understanding of the disorder’s pathophysiology and the development of specific treatments. With a focus on addressing the unmet medical needs of individuals affected by TD, the market strives to offer effective and well-tolerated therapeutic options. Stakeholders in this dynamic landscape need to stay abreast of key market insights to navigate challenges and capitalize on emerging opportunities.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The US Tardive Dyskinesia therapeutics market operates in a dynamic environment shaped by factors such as evolving research landscapes, regulatory developments, and patient advocacy initiatives. Understanding these dynamics is crucial for stakeholders to adapt strategies, navigate challenges, and capitalize on emerging opportunities in this complex therapeutic space.
Regional Analysis:
Regional variations in the prevalence of TD, access to healthcare, and treatment-seeking behavior influence the market dynamics. Analyzing regional trends allows stakeholders to tailor approaches based on specific geographical considerations.
Competitive Landscape:
Leading Companies in the US Tardive Dyskinesia Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
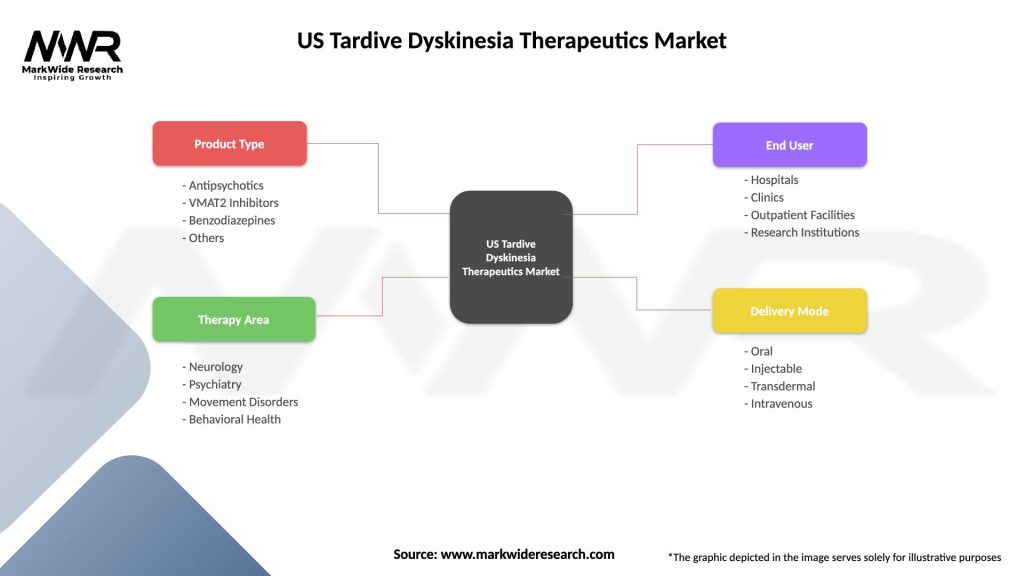
Segmentation:
The US Tardive Dyskinesia therapeutics market can be segmented based on various factors, including:
Segmentation enhances the understanding of market dynamics, allowing for targeted approaches in therapeutic development and patient care.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
The US Tardive Dyskinesia therapeutics market offers several benefits for industry participants and stakeholders:
SWOT Analysis:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has influenced the US Tardive Dyskinesia therapeutics market in various ways:
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The US Tardive Dyskinesia therapeutics market is poised for continued growth, driven by advancements in research, ongoing clinical trials, and a focus on personalized treatment approaches. The future landscape will be shaped by innovations in pharmacological and neuromodulation therapies, precision medicine applications, and collaborative efforts to address the multifaceted challenges associated with TD. As the field evolves, stakeholders are encouraged to stay abreast of emerging trends, regulatory developments, and patient perspectives to contribute to the advancement of TD therapeutics.
Conclusion:
The US Tardive Dyskinesia therapeutics market represents a dynamic and evolving sector within the broader landscape of neurological disorders and mental health. With a growing understanding of TD’s pathophysiology, ongoing research initiatives, and a focus on patient-centric care, the market is positioned for transformative advancements. Despite challenges related to limited treatment options and complex regulatory landscapes, the commitment to addressing unmet medical needs and improving patient outcomes underscores the resilience and potential of the TD therapeutics market. By fostering collaborations, embracing innovation, and prioritizing long-term safety, stakeholders can contribute to shaping a future where effective and well-tolerated treatments enhance the lives of individuals affected by Tardive Dyskinesia.
What is Tardive Dyskinesia Therapeutics?
Tardive Dyskinesia Therapeutics refers to treatments specifically designed to manage and alleviate the symptoms of tardive dyskinesia, a movement disorder often caused by long-term use of antipsychotic medications. These therapeutics aim to improve patients’ quality of life by reducing involuntary movements and associated discomfort.
What are the key players in the US Tardive Dyskinesia Therapeutics Market?
Key players in the US Tardive Dyskinesia Therapeutics Market include companies such as Teva Pharmaceutical Industries, Neurocrine Biosciences, and AbbVie, which are actively involved in developing and marketing treatments for this condition, among others.
What are the growth factors driving the US Tardive Dyskinesia Therapeutics Market?
The growth of the US Tardive Dyskinesia Therapeutics Market is driven by an increasing prevalence of mental health disorders requiring antipsychotic treatment, heightened awareness of tardive dyskinesia among healthcare providers, and advancements in therapeutic options that offer better efficacy and safety profiles.
What challenges does the US Tardive Dyskinesia Therapeutics Market face?
The US Tardive Dyskinesia Therapeutics Market faces challenges such as the high cost of new therapies, potential side effects associated with treatments, and the need for ongoing research to better understand the condition and develop more effective solutions.
What opportunities exist in the US Tardive Dyskinesia Therapeutics Market?
Opportunities in the US Tardive Dyskinesia Therapeutics Market include the development of novel therapies that target specific symptoms, the potential for combination treatments, and the expansion of patient access through telemedicine and improved healthcare policies.
What trends are shaping the US Tardive Dyskinesia Therapeutics Market?
Trends shaping the US Tardive Dyskinesia Therapeutics Market include a focus on personalized medicine, increased investment in research and development for innovative treatments, and the integration of digital health solutions to monitor and manage symptoms more effectively.
US Tardive Dyskinesia Therapeutics Market
| Segmentation Details | Description |
|---|---|
| Product Type | Antipsychotics, VMAT2 Inhibitors, Benzodiazepines, Others |
| Therapy Area | Neurology, Psychiatry, Movement Disorders, Behavioral Health |
| End User | Hospitals, Clinics, Outpatient Facilities, Research Institutions |
| Delivery Mode | Oral, Injectable, Transdermal, Intravenous |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the US Tardive Dyskinesia Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at