444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview:
The US Mycoplasma Testing market is a crucial segment within the diagnostic testing industry, specializing in the detection and identification of mycoplasma infections. Mycoplasma, a type of bacteria lacking a cell wall, can cause various infections in humans, animals, and cell cultures. The market’s focus on accurate and efficient testing contributes to the overall management of mycoplasma-related health concerns.
Meaning:
Mycoplasma testing involves diagnostic procedures aimed at detecting and identifying mycoplasma infections. This type of testing is essential in research, healthcare, and biopharmaceutical manufacturing to ensure the integrity and safety of cell cultures and biological products.
Executive Summary:
The US Mycoplasma Testing market is characterized by its significance in maintaining the quality and safety of biological products, research materials, and healthcare settings. The market’s growth is driven by the increasing awareness of mycoplasma contamination risks and the importance of reliable testing methods.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The dynamics of the US Mycoplasma Testing market are influenced by factors such as regulatory requirements, advancements in diagnostic technologies, the prevalence of mycoplasma contamination incidents, and the evolving needs of end-users. Staying abreast of these dynamics is essential for companies operating in this market.
Regional Analysis:
The distribution and prevalence of mycoplasma contamination incidents may vary regionally within the United States. Regional analysis helps tailor testing strategies to address specific challenges and risks associated with mycoplasma in different geographic areas.
Competitive Landscape:
Leading Companies in US Mycoplasma Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation:
The Mycoplasma Testing market can be segmented based on:
Segmentation allows for a targeted approach in meeting the specific testing needs of different industries and end-users, tailoring testing solutions accordingly.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Understanding these internal and external factors through a SWOT analysis assists industry participants in formulating strategic decisions and adapting to market dynamics.
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has underscored the importance of robust diagnostic testing infrastructure. While the focus has been on addressing the pandemic, the mycoplasma testing market has adapted by incorporating lessons learned from the pandemic response into its testing strategies and protocols.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The future outlook for the US Mycoplasma Testing market is optimistic, driven by the increasing emphasis on quality control in biopharmaceutical manufacturing, the importance of research integrity, and the need for accurate diagnostic solutions in healthcare. Ongoing advancements in testing technologies and a focus on user-friendly testing solutions will shape the market’s trajectory.
Conclusion:
In conclusion, the US Mycoplasma Testing market plays a pivotal role in maintaining the quality and safety of biopharmaceuticals, preserving research integrity, and enabling accurate diagnoses in healthcare. The market’s dynamics are influenced by regulatory standards, technological advancements, and the evolving needs of end-users. As the importance of mycoplasma testing continues to grow, industry participants are poised to contribute to advancements in testing methods, ensuring the market’s positive impact on public health and scientific research.
What is Mycoplasma Testing?
Mycoplasma Testing refers to the diagnostic procedures used to detect mycoplasma infections, which are caused by a group of bacteria lacking a cell wall. These tests are crucial in various fields, including clinical diagnostics, research, and biopharmaceutical manufacturing, to ensure the quality and safety of products.
What are the key players in the US Mycoplasma Testing Market?
Key players in the US Mycoplasma Testing Market include Thermo Fisher Scientific, Merck KGaA, and BioMérieux, among others. These companies are known for their innovative testing solutions and contributions to the development of mycoplasma detection technologies.
What are the growth factors driving the US Mycoplasma Testing Market?
The growth of the US Mycoplasma Testing Market is driven by the increasing demand for quality control in biopharmaceutical manufacturing, rising prevalence of mycoplasma infections, and advancements in molecular diagnostic technologies. Additionally, the expansion of research activities in microbiology is contributing to market growth.
What challenges does the US Mycoplasma Testing Market face?
The US Mycoplasma Testing Market faces challenges such as the high costs associated with advanced testing technologies and the complexity of testing procedures. Furthermore, the lack of standardized testing methods can lead to inconsistent results, impacting the reliability of diagnostics.
What opportunities exist in the US Mycoplasma Testing Market?
Opportunities in the US Mycoplasma Testing Market include the development of rapid testing kits and the integration of automation in testing processes. Additionally, increasing investments in research and development for novel therapeutic solutions present significant growth potential.
What trends are shaping the US Mycoplasma Testing Market?
Trends in the US Mycoplasma Testing Market include the rising adoption of PCR-based testing methods and the growing focus on regulatory compliance in biopharmaceutical production. Moreover, there is an increasing emphasis on the use of next-generation sequencing technologies for more accurate detection.
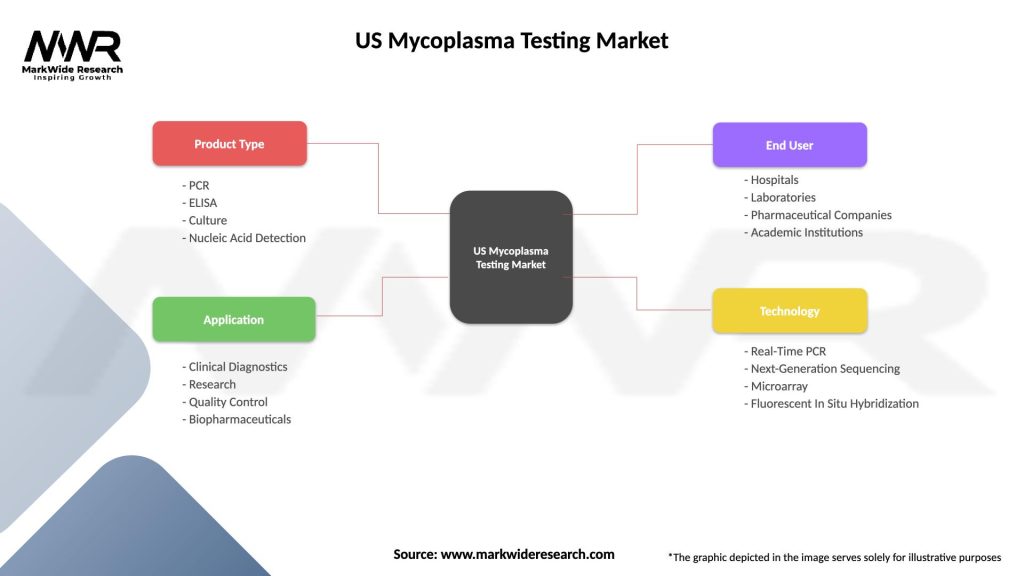
US Mycoplasma Testing Market
| Segmentation Details | Description |
|---|---|
| Product Type | PCR, ELISA, Culture, Nucleic Acid Detection |
| Application | Clinical Diagnostics, Research, Quality Control, Biopharmaceuticals |
| End User | Hospitals, Laboratories, Pharmaceutical Companies, Academic Institutions |
| Technology | Real-Time PCR, Next-Generation Sequencing, Microarray, Fluorescent In Situ Hybridization |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in US Mycoplasma Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at