444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market has witnessed significant growth in recent years. DBS is a neurosurgical procedure that involves the implantation of a medical device, often referred to as a “brain pacemaker,” to alleviate the symptoms of Parkinson’s disease. It works by delivering electrical impulses to specific areas of the brain, which helps regulate abnormal brain activity and improves motor function in patients.
Meaning
Deep Brain Stimulation (DBS) is a therapeutic technique used to treat various neurological disorders, including Parkinson’s disease. It involves the implantation of a neurostimulator device, similar to a pacemaker, into specific regions of the brain. This device delivers electrical stimulation to targeted areas, helping to reduce symptoms such as tremors, stiffness, and mobility issues associated with Parkinson’s disease.
Executive Summary
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market has experienced substantial growth in recent years. The rising prevalence of Parkinson’s disease, coupled with advancements in neurosurgical techniques and the increasing adoption of DBS as a treatment option, has contributed to market expansion. This report provides comprehensive insights into the market, including key drivers, restraints, opportunities, and competitive landscape analysis.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
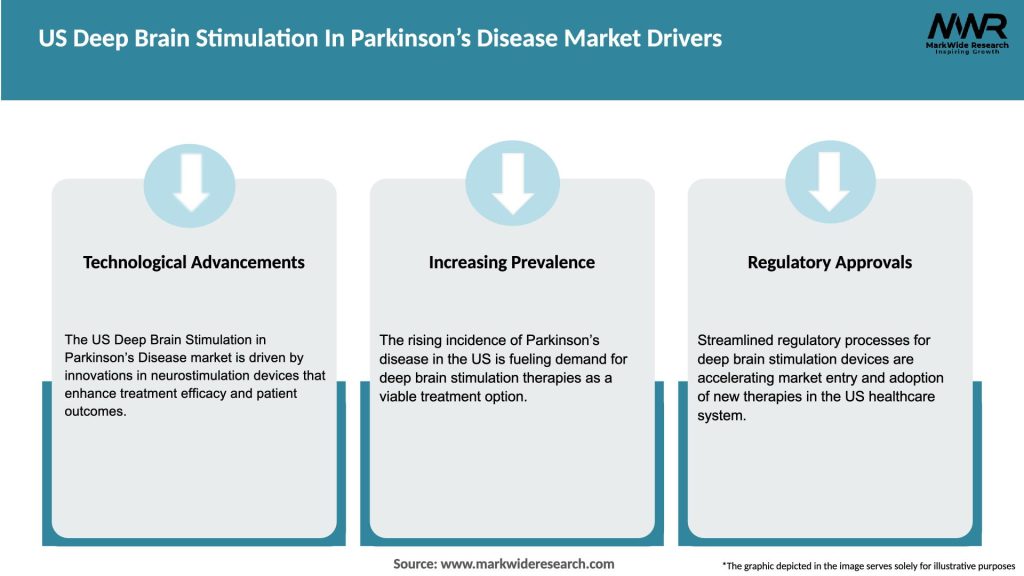
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market is characterized by dynamic factors that influence its growth trajectory. These dynamics include technological advancements, changes in patient preferences, regulatory landscape, reimbursement policies, and competitive market forces. Understanding these dynamics is essential for industry participants to adapt to market trends and make informed business decisions.
Regional Analysis
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market can be analyzed based on regional segmentation. The market is likely to be concentrated in regions with a higher prevalence of Parkinson’s disease, well-established healthcare infrastructure, and specialized neurosurgical centers. Major metropolitan areas, academic medical centers, and research institutions often serve as hubs for DBS procedures and clinical trials.
Competitive Landscape
Leading Companies in the US Deep Brain Stimulation in Parkinson’s Disease Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
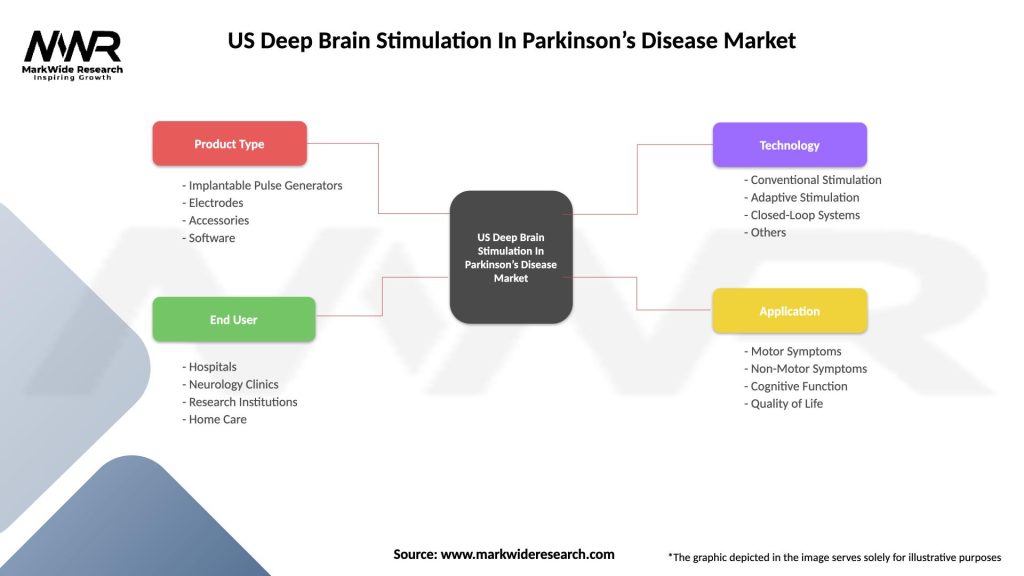
Segmentation
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market can be segmented based on various factors, including:
Segmentation allows for a better understanding of the market dynamics, target audience, and specific needs of different customer segments.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis provides a comprehensive overview of the US Deep Brain Stimulation (DBS) in Parkinson’s Disease market.
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the US Deep Brain Stimulation (DBS) in Parkinson’s Disease market. The pandemic led to disruptions in healthcare services, including elective surgeries and non-emergency procedures. This resulted in delays or cancellations of DBS surgeries and follow-up appointments, impacting market growth.
However, the pandemic also highlighted the importance of alternative treatment options like DBS. Parkinson’s disease patients, especially those at advanced stages, may face increased challenges and susceptibility to infections. The need for effective symptom management and improved quality of life through DBS remains crucial, and the market is expected to rebound as the healthcare system recovers from the pandemic’s impact.
Key Industry Developments
Analyst Suggestions
Future Outlook
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market is expected to witness steady growth in the coming years. The rising prevalence of Parkinson’s disease, increasing acceptance of DBS as a treatment option, technological advancements, and expanding indications contribute to the positive market outlook. However, challenges such as cost barriers, surgical risks, and limited access to specialized centers need to be addressed to ensure wider adoption and market expansion.
The market is likely to see continued innovation in DBS devices, personalized medicine approaches, and integration of artificial intelligence. Collaborations between industry players, research institutions, and healthcare providers will drive advancements in technology and expand the applications of DBS. The market’s future growth will be influenced by factors such as regulatory developments, reimbursement policies, and the evolving healthcare landscape.
Conclusion
The US Deep Brain Stimulation (DBS) in Parkinson’s Disease market has experienced significant growth and is expected to continue its upward trajectory. DBS offers an effective treatment option for Parkinson’s disease patients who do not respond adequately to medication alone. Technological advancements, growing awareness and acceptance, and favorable reimbursement policies are key drivers of market expansion. While the market presents lucrative opportunities, challenges such as high costs, surgical risks, and limited access to specialized centers need to be addressed. Continued investment in research and development, collaborations between industry stakeholders and research institutions, and patient-centric approaches will drive future market growth.
What is US Deep Brain Stimulation In Parkinson’s Disease?
US Deep Brain Stimulation In Parkinson’s Disease refers to a neurosurgical procedure that involves implanting electrodes in specific brain regions to alleviate symptoms of Parkinson’s disease, such as tremors and rigidity. This treatment is often considered when medication is no longer effective.
Who are the key players in the US Deep Brain Stimulation In Parkinson’s Disease market?
Key players in the US Deep Brain Stimulation In Parkinson’s Disease market include Medtronic, Boston Scientific, and Abbott, among others. These companies are involved in the development and manufacturing of advanced deep brain stimulation devices.
What are the growth factors driving the US Deep Brain Stimulation In Parkinson’s Disease market?
The growth of the US Deep Brain Stimulation In Parkinson’s Disease market is driven by the increasing prevalence of Parkinson’s disease, advancements in neuromodulation technologies, and a growing awareness of treatment options among patients and healthcare providers.
What challenges does the US Deep Brain Stimulation In Parkinson’s Disease market face?
Challenges in the US Deep Brain Stimulation In Parkinson’s Disease market include high costs associated with the procedure, potential surgical risks, and varying patient responses to treatment. Additionally, there may be regulatory hurdles that affect device approval.
What future opportunities exist in the US Deep Brain Stimulation In Parkinson’s Disease market?
Future opportunities in the US Deep Brain Stimulation In Parkinson’s Disease market include the development of more advanced and patient-specific devices, integration of artificial intelligence for better outcomes, and expanding applications of deep brain stimulation for other neurological disorders.
What trends are shaping the US Deep Brain Stimulation In Parkinson’s Disease market?
Trends shaping the US Deep Brain Stimulation In Parkinson’s Disease market include the increasing use of minimally invasive surgical techniques, the rise of remote patient monitoring technologies, and ongoing research into the long-term effects of deep brain stimulation on quality of life.
US Deep Brain Stimulation In Parkinson’s Disease Market
| Segmentation Details | Description |
|---|---|
| Product Type | Implantable Pulse Generators, Electrodes, Accessories, Software |
| End User | Hospitals, Neurology Clinics, Research Institutions, Home Care |
| Technology | Conventional Stimulation, Adaptive Stimulation, Closed-Loop Systems, Others |
| Application | Motor Symptoms, Non-Motor Symptoms, Cognitive Function, Quality of Life |
Leading Companies in the US Deep Brain Stimulation in Parkinson’s Disease Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at