444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The US Clinical Oncology Next Generation Sequencing (NGS) Market is at the forefront of revolutionizing cancer diagnosis, treatment, and personalized medicine. Next-generation sequencing technologies enable comprehensive genomic profiling of tumors, providing valuable insights into the molecular mechanisms of cancer, identifying actionable genetic alterations, and guiding targeted therapies. With advancements in NGS platforms, bioinformatics tools, and data analysis techniques, the US clinical oncology NGS market is experiencing rapid growth, driven by the increasing prevalence of cancer, demand for precision medicine, and investments in genomic research and oncology diagnostics.
Meaning
Clinical oncology next-generation sequencing (NGS) refers to advanced genomic sequencing techniques used in cancer diagnosis, prognosis, and treatment decision-making. NGS enables the high-throughput analysis of DNA, RNA, and other biomolecules in tumor samples, allowing clinicians to identify genetic mutations, copy number alterations, and gene expression patterns associated with cancer development, progression, and drug response. By integrating NGS data with clinical information, oncologists can tailor treatment strategies, monitor disease evolution, and improve patient outcomes in the US clinical oncology NGS market.
Executive Summary
The US Clinical Oncology Next Generation Sequencing Market is witnessing robust growth fueled by the expanding applications of NGS in cancer care, growing adoption of precision medicine approaches, and advancements in genomic technologies and bioinformatics. Key market players are investing in R&D, strategic collaborations, and product innovations to address unmet clinical needs, enhance test accuracy, and accelerate the translation of genomic discoveries into clinical practice. With the rise of targeted therapies, immunotherapies, and liquid biopsy assays, the US clinical oncology NGS market is poised for further expansion and transformation in the coming years.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The US Clinical Oncology Next Generation Sequencing Market operates in a dynamic environment shaped by technological advancements, regulatory trends, healthcare policies, and market dynamics. Key drivers, restraints, and opportunities influence market growth, adoption rates, and competitive dynamics, requiring stakeholders to navigate uncertainties, address challenges, and capitalize on emerging trends to succeed in the rapidly evolving US market.
Regional Analysis
The US Clinical Oncology Next Generation Sequencing Market spans diverse geographic regions with varying healthcare infrastructure, research capabilities, and patient populations. While major metropolitan areas and academic medical centers serve as hubs for NGS-based oncology diagnostics and research, regional disparities in access to genetic testing, clinical trials, and precision oncology services pose challenges and opportunities for market expansion and equity across the US healthcare landscape.
Competitive Landscape
Leading Companies in US Clinical Oncology Next Generation Sequencing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
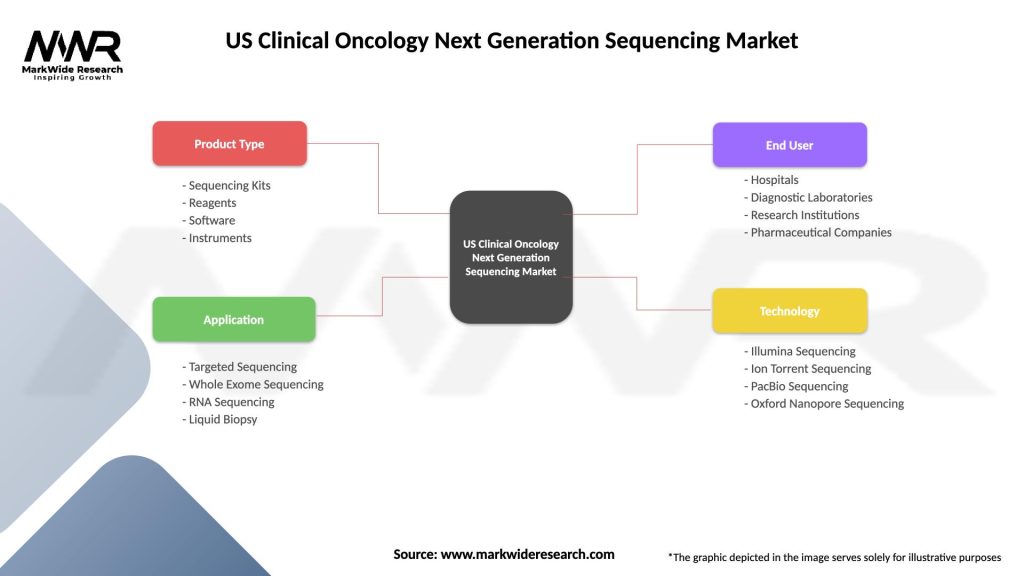
Segmentation
The US Clinical Oncology Next Generation Sequencing Market can be segmented based on various factors, including:
Segmentation enables providers to offer tailored NGS-based solutions to meet the specific clinical and research needs of oncologists, pathologists, and researchers in the US market.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT analysis of the US Clinical Oncology Next Generation Sequencing Market reveals the following insights:
Understanding these factors helps stakeholders navigate market dynamics, capitalize on strengths, mitigate weaknesses, leverage opportunities, and address threats in the dynamic US clinical oncology NGS market landscape.
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has both accelerated and reshaped the US Clinical Oncology Next Generation Sequencing Market. While the pandemic has disrupted healthcare delivery, research activities, and clinical trials, it has also highlighted the importance of genomic diagnostics, telehealth, and digital health technologies in cancer care, driving innovation, adoption, and investment in NGS-based oncology solutions in the US market.
Key Industry Developments
Analyst Suggestions
Future Outlook
The US Clinical Oncology Next Generation Sequencing Market is poised for exponential growth and transformation driven by technological advancements, clinical innovation, and healthcare digitization. As NGS technologies continue to evolve, the integration of genomic data into routine oncology practice, real-world evidence generation, and population health initiatives will reshape cancer care delivery, accelerate therapeutic innovation, and improve patient outcomes in the dynamic and competitive US market.
Conclusion
The US Clinical Oncology Next Generation Sequencing Market represents a transformative force in cancer diagnostics, precision medicine, and personalized oncology care. With its ability to unravel the complexity of cancer genomes, predict treatment response, and guide therapeutic decision-making, NGS technologies empower clinicians, researchers, and patients to confront cancer with precision, knowledge, and hope. By harnessing the power of genomics, bioinformatics, and collaborative innovation, stakeholders can advance the frontiers of cancer science, redefine the standard of care, and unlock new possibilities for conquering cancer in the US clinical oncology NGS market landscape.
What is Clinical Oncology Next Generation Sequencing?
Clinical Oncology Next Generation Sequencing refers to advanced genomic sequencing technologies used to analyze cancer-related genes, helping in the diagnosis, treatment planning, and monitoring of cancer therapies.
What are the key players in the US Clinical Oncology Next Generation Sequencing Market?
Key players in the US Clinical Oncology Next Generation Sequencing Market include Illumina, Thermo Fisher Scientific, and Agilent Technologies, among others.
What are the main drivers of growth in the US Clinical Oncology Next Generation Sequencing Market?
The growth of the US Clinical Oncology Next Generation Sequencing Market is driven by the increasing prevalence of cancer, advancements in sequencing technologies, and the rising demand for personalized medicine.
What challenges does the US Clinical Oncology Next Generation Sequencing Market face?
Challenges in the US Clinical Oncology Next Generation Sequencing Market include high costs of sequencing technologies, regulatory hurdles, and the need for skilled professionals to interpret complex genomic data.
What opportunities exist in the US Clinical Oncology Next Generation Sequencing Market?
Opportunities in the US Clinical Oncology Next Generation Sequencing Market include the development of new biomarkers for cancer treatment, expansion into emerging markets, and integration of artificial intelligence for data analysis.
What trends are shaping the US Clinical Oncology Next Generation Sequencing Market?
Trends in the US Clinical Oncology Next Generation Sequencing Market include the increasing adoption of liquid biopsy techniques, the rise of multi-omics approaches, and the growing emphasis on data sharing and collaboration in research.
US Clinical Oncology Next Generation Sequencing Market
| Segmentation Details | Description |
|---|---|
| Product Type | Sequencing Kits, Reagents, Software, Instruments |
| Application | Targeted Sequencing, Whole Exome Sequencing, RNA Sequencing, Liquid Biopsy |
| End User | Hospitals, Diagnostic Laboratories, Research Institutions, Pharmaceutical Companies |
| Technology | Illumina Sequencing, Ion Torrent Sequencing, PacBio Sequencing, Oxford Nanopore Sequencing |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in US Clinical Oncology Next Generation Sequencing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at