444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The ultra-low temperature freezer market for COVID-19 vaccines is witnessing significant growth due to the urgent need for reliable storage solutions to preserve vaccines at extremely low temperatures. These freezers play a critical role in maintaining the efficacy of vaccines, particularly mRNA vaccines like those developed for COVID-19. As countries worldwide ramp up vaccination efforts, the demand for ultra-low temperature freezers continues to rise, presenting lucrative opportunities for market players.
Meaning
Ultra-low temperature freezers are specialized refrigeration units designed to store vaccines and biological samples at ultra-low temperatures, typically below -70°C (-94°F). These freezers utilize advanced cooling technologies to maintain stable temperature conditions, ensuring the integrity and efficacy of temperature-sensitive products. In the context of COVID-19 vaccines, ultra-low temperature freezers are essential for preserving mRNA vaccines, which degrade rapidly at higher temperatures.
Executive Summary
The ultra-low temperature freezer market for COVID-19 vaccines is driven by the global vaccination campaign to combat the COVID-19 pandemic. Key market insights reveal the critical role of ultra-low temperature freezers in vaccine distribution and storage logistics. Market players are focusing on innovation and capacity expansion to meet the growing demand for reliable cold storage solutions. However, challenges such as supply chain constraints and regulatory compliance pose significant hurdles to market growth.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The ultra-low temperature freezer market for COVID-19 vaccines operates within a dynamic landscape shaped by evolving vaccine distribution strategies, technological advancements, regulatory frameworks, and global health priorities. Understanding these dynamics is essential for stakeholders to navigate market uncertainties and capitalize on emerging opportunities.
Regional Analysis
Competitive Landscape
Leading Companies in the Ultra-low Temperature Freezer for COVID-19 Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
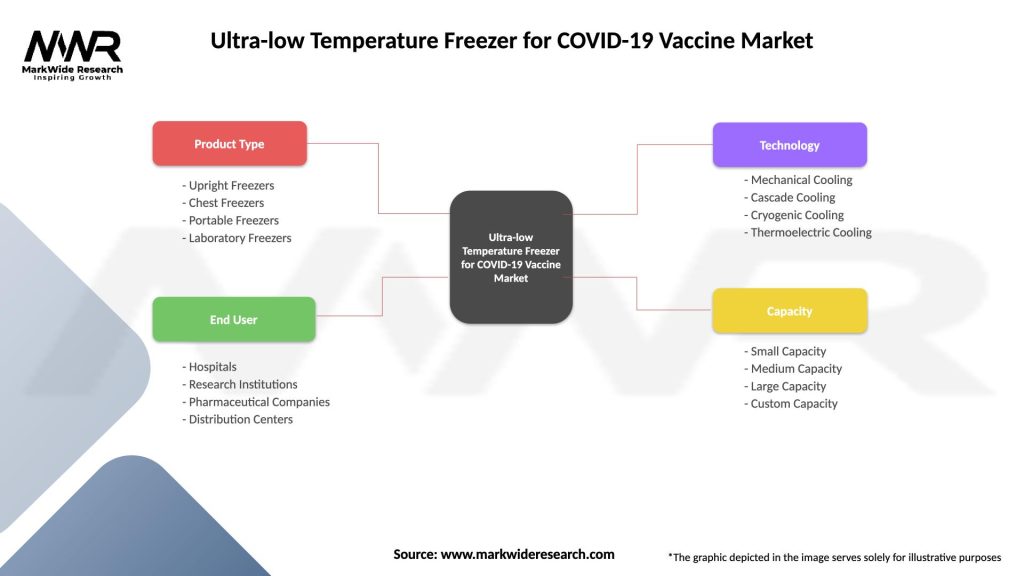
Segmentation
The ultra-low temperature freezer market can be segmented based on freezer type, capacity, end-user, and geography. Segmenting the market provides insights into customer preferences and market dynamics, enabling manufacturers to tailor their product offerings and marketing strategies accordingly.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Understanding these factors through a SWOT analysis helps stakeholders identify strategic priorities, mitigate risks, and capitalize on market opportunities.
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic accelerated the demand for ultra-low temperature freezers, particularly with the rollout of mRNA-based COVID-19 vaccines requiring stringent temperature control. Key impacts of COVID-19 on the market include:
Key Industry Developments
Analyst Suggestions
Future Outlook
The ultra-low temperature freezer market for COVID-19 vaccines is poised for sustained growth in the post-pandemic era, driven by continued vaccination efforts, emerging infectious disease threats, and advancements in vaccine development technologies. Key trends such as technological innovation, sustainability initiatives, and collaborative partnerships will shape the market landscape, presenting opportunities for stakeholders to innovate, adapt, and thrive in a dynamic healthcare environment.
Conclusion
The ultra-low temperature freezer market for COVID-19 vaccines plays a pivotal role in ensuring the effective storage, distribution, and administration of vaccines critical to global public health. Despite challenges posed by the COVID-19 pandemic, the market has demonstrated resilience and adaptability, with stakeholders collaborating to overcome supply chain disruptions and address evolving vaccine storage requirements. Moving forward, stakeholders must remain vigilant and proactive in addressing key challenges such as supply chain resilience, regulatory compliance, and technological innovation. By investing in scalable, sustainable solutions and fostering collaboration across the vaccine supply chain, stakeholders can ensure equitable access to vaccines and contribute to global health security.
In conclusion, the ultra-low temperature freezer market for COVID-19 vaccines is witnessing unprecedented growth and demand driven by the urgent need for reliable vaccine storage solutions. These freezers play a vital role in preserving vaccine efficacy and facilitating mass vaccination campaigns worldwide. Despite challenges such as supply chain disruptions and regulatory complexities, stakeholders are innovating and collaborating to overcome obstacles and ensure the efficient distribution of vaccines. Looking ahead, continued investments in technology, sustainability, and collaborative partnerships will be essential to address emerging healthcare challenges and support global vaccination efforts.
What is Ultra-low Temperature Freezer for COVID-19 Vaccine?
An ultra-low temperature freezer for COVID-19 vaccine is a specialized refrigeration unit designed to store vaccines at extremely low temperatures, typically between minus seventy and minus eighty degrees Celsius. These freezers are essential for maintaining the efficacy of mRNA vaccines, which require strict temperature control during storage and transportation.
What are the key players in the Ultra-low Temperature Freezer for COVID-19 Vaccine Market?
Key players in the ultra-low temperature freezer for COVID-19 vaccine market include companies like Thermo Fisher Scientific, Panasonic Healthcare, and Haier Biomedical, among others. These companies are known for their advanced refrigeration technologies and have established a strong presence in the healthcare sector.
What are the growth factors driving the Ultra-low Temperature Freezer for COVID-19 Vaccine Market?
The growth of the ultra-low temperature freezer for COVID-19 vaccine market is driven by the increasing demand for effective vaccine storage solutions, the rise in vaccination campaigns globally, and advancements in refrigeration technology. Additionally, the need for reliable cold chain logistics is crucial for vaccine distribution.
What challenges does the Ultra-low Temperature Freezer for COVID-19 Vaccine Market face?
Challenges in the ultra-low temperature freezer for COVID-19 vaccine market include high operational costs, limited availability of energy-efficient models, and the need for regular maintenance. Furthermore, ensuring consistent power supply in remote areas can pose significant logistical issues.
What opportunities exist in the Ultra-low Temperature Freezer for COVID-19 Vaccine Market?
Opportunities in the ultra-low temperature freezer for COVID-19 vaccine market include the expansion of vaccine distribution networks, increasing investments in healthcare infrastructure, and the development of innovative storage solutions. The ongoing research into new vaccines also presents potential growth avenues.
What trends are shaping the Ultra-low Temperature Freezer for COVID-19 Vaccine Market?
Trends in the ultra-low temperature freezer for COVID-19 vaccine market include the integration of IoT technology for real-time monitoring, the development of portable freezer units, and a focus on sustainability in manufacturing processes. These trends aim to enhance efficiency and reliability in vaccine storage.
Ultra-low Temperature Freezer for COVID-19 Vaccine Market
| Segmentation Details | Description |
|---|---|
| Product Type | Upright Freezers, Chest Freezers, Portable Freezers, Laboratory Freezers |
| End User | Hospitals, Research Institutions, Pharmaceutical Companies, Distribution Centers |
| Technology | Mechanical Cooling, Cascade Cooling, Cryogenic Cooling, Thermoelectric Cooling |
| Capacity | Small Capacity, Medium Capacity, Large Capacity, Custom Capacity |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Ultra-low Temperature Freezer for COVID-19 Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at