444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The TIL (Tumor-Infiltrating Lymphocytes) therapy market is experiencing remarkable growth, fueled by the promising potential of personalized immunotherapy in cancer treatment. TIL therapy involves isolating, expanding, and reinfusing a patient’s own immune cells, specifically T cells, which have infiltrated the tumor microenvironment, to target and eliminate cancer cells. This innovative approach harnesses the power of the immune system to recognize and attack cancer, offering new hope for patients with advanced or refractory malignancies. The market for TIL therapy is driven by factors such as increasing prevalence of cancer, advancements in cell therapy technologies, and growing investments in oncology research and development.
Meaning
TIL therapy is a form of adoptive cell therapy (ACT) that involves isolating tumor-infiltrating lymphocytes (TILs) from a patient’s tumor tissue, expanding these TILs ex vivo (outside the body) to large numbers, and then reinfusing them back into the patient. TILs are a subset of immune cells that have migrated into the tumor and have the potential to recognize and attack cancer cells. By enhancing the activity and specificity of these TILs, TIL therapy aims to boost the body’s natural immune response against cancer, offering a personalized and targeted approach to cancer treatment.
Executive Summary
The TIL therapy market is characterized by groundbreaking scientific discoveries, rapid technological advancements, and strategic collaborations among biopharmaceutical companies, academic research institutions, and healthcare providers. Market players are focused on optimizing manufacturing processes, expanding clinical indications, and navigating regulatory pathways to bring TIL therapy to a wider patient population and address unmet needs in oncology.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The TIL therapy market is influenced by various factors, including scientific breakthroughs, clinical trial outcomes, regulatory policies, reimbursement landscape, and market competition. Key dynamics shaping the market include:
Regional Analysis
The global TIL therapy market is segmented into regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America dominates the market, attributed to factors such as robust research infrastructure, favorable regulatory environment, and high prevalence of cancer, driving demand for innovative cancer therapies like TIL therapy.
Competitive Landscape
Leading Companies in the TIL Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
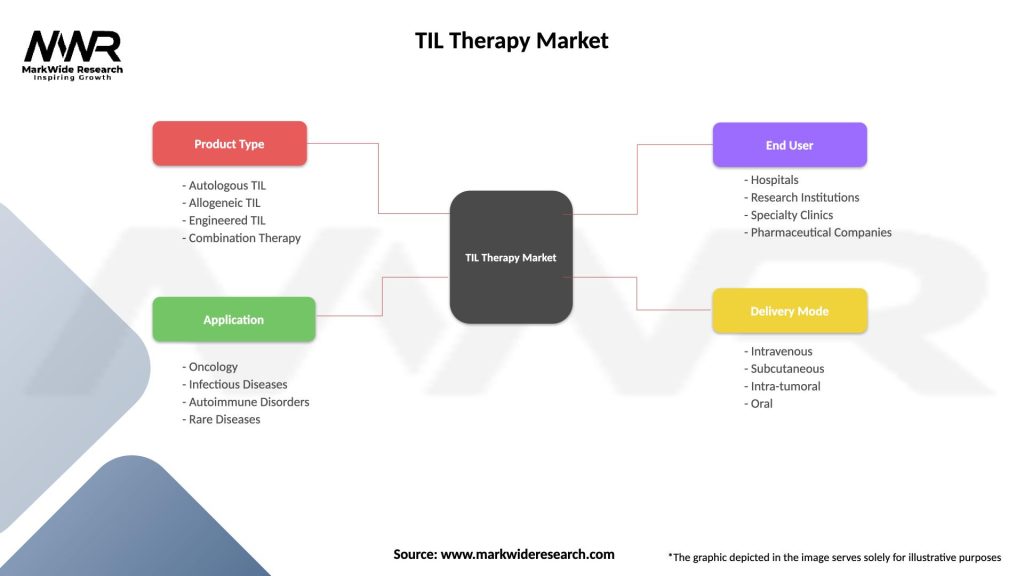
Segmentation
The TIL therapy market can be segmented based on cancer type, therapy type, application, end-user, and geography. By cancer type, the market includes melanoma, cervical cancer, non-small cell lung cancer, and other solid tumors. By therapy type, the market comprises autologous TIL therapy and allogeneic TIL therapy. By application, the market covers adjuvant therapy, frontline therapy, salvage therapy, and combination therapy. By end-user, the market includes hospitals, cancer centers, academic research institutes, and outpatient clinics.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has underscored the importance of immunotherapy and personalized medicine in oncology, highlighting the need for innovative treatments that offer durable responses and reduced reliance on traditional cytotoxic chemotherapy. TIL therapy, with its potential for long-term remissions and immune memory, has emerged as a promising therapeutic option for cancer patients amidst the challenges of the pandemic.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the TIL therapy market is highly promising, driven by the increasing recognition of the immune system’s role in cancer surveillance and eradication, as well as the expanding applications of TIL therapy across diverse cancer types and clinical settings. As the field of cancer immunotherapy continues to evolve, fueled by scientific discoveries, technological innovations, and collaborative efforts, the market for TIL therapy is expected to witness sustained growth and transformative impact on cancer care and patient outcomes.
Conclusion
In conclusion, TIL therapy represents a paradigm shift in cancer treatment, offering personalized immunotherapy solutions that harness the body’s own immune defenses to target and eliminate cancer cells. Despite challenges such as manufacturing complexity and regulatory hurdles, TIL therapy holds immense promise for patients with advanced or refractory malignancies, providing new hope for durable responses, long-term survival, and improved quality of life in the fight against cancer.
What is TIL Therapy?
TIL Therapy, or Tumor-Infiltrating Lymphocyte Therapy, is an innovative cancer treatment that utilizes the patient’s own immune cells to target and destroy tumor cells. This approach focuses on enhancing the body’s natural immune response against cancerous growths.
What are the key companies in the TIL Therapy Market?
Key companies in the TIL Therapy Market include Iovance Biotherapeutics, Adaptimmune Therapeutics, and Novartis, which are actively involved in developing and commercializing TIL therapies for various cancer types, among others.
What are the growth factors driving the TIL Therapy Market?
The TIL Therapy Market is driven by increasing cancer prevalence, advancements in immunotherapy, and growing investments in cancer research. Additionally, the rising demand for personalized medicine is propelling the development of TIL therapies.
What challenges does the TIL Therapy Market face?
The TIL Therapy Market faces challenges such as high treatment costs, complex manufacturing processes, and regulatory hurdles. These factors can limit accessibility and slow down the adoption of TIL therapies in clinical settings.
What opportunities exist in the TIL Therapy Market?
Opportunities in the TIL Therapy Market include expanding research into combination therapies, increasing collaborations between biotech firms and research institutions, and the potential for TIL therapies to be used in treating a wider range of cancers.
What trends are emerging in the TIL Therapy Market?
Emerging trends in the TIL Therapy Market include the integration of artificial intelligence in treatment planning, advancements in cell manufacturing technologies, and a focus on improving patient outcomes through personalized treatment approaches.
TIL Therapy Market
| Segmentation Details | Description |
|---|---|
| Product Type | Autologous TIL, Allogeneic TIL, Engineered TIL, Combination Therapy |
| Application | Oncology, Infectious Diseases, Autoimmune Disorders, Rare Diseases |
| End User | Hospitals, Research Institutions, Specialty Clinics, Pharmaceutical Companies |
| Delivery Mode | Intravenous, Subcutaneous, Intra-tumoral, Oral |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the TIL Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at