444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
Thromboxane A2 (TXA2) ELISA kits are vital tools used in biomedical research and clinical diagnostics to quantify thromboxane A2 levels accurately. These kits enable the detection and measurement of TXA2, a potent vasoconstrictor and platelet aggregator, implicated in various cardiovascular and inflammatory diseases. The TXA2 ELISA kit market serves researchers, clinicians, and pharmaceutical companies engaged in studying TXA2 biology, drug development, and patient management.
Meaning:
Thromboxane A2 (TXA2) is a bioactive lipid mediator synthesized from arachidonic acid by the enzyme thromboxane synthase. It plays a crucial role in hemostasis, platelet activation, and vasoconstriction, contributing to cardiovascular homeostasis and inflammation regulation. TXA2 ELISA kits employ enzyme-linked immunosorbent assay (ELISA) technology to quantify TXA2 levels in biological samples, providing valuable insights into thromboxane biology, disease pathogenesis, and therapeutic interventions.
Executive Summary:
The TXA2 ELISA kit market is witnessing steady growth due to the rising incidence of cardiovascular diseases, inflammatory disorders, and thrombotic events globally. Increasing research investments, technological advancements, and clinical utility drive market expansion. However, challenges such as assay standardization, sample variability, and regulatory compliance impact market dynamics. Strategic collaborations, product innovations, and quality assurance measures are essential for market players to maintain competitiveness and meet customer demands.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics
Key dynamics influencing the TXA2 ELISA Kit Market include:
Regional Analysis
The TXA2 ELISA Kit Market shows varied trends and opportunities across different regions:
Competitive Landscape
Leading Companies in the Thromboxane A2 (TXA2) ELISA Kit Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
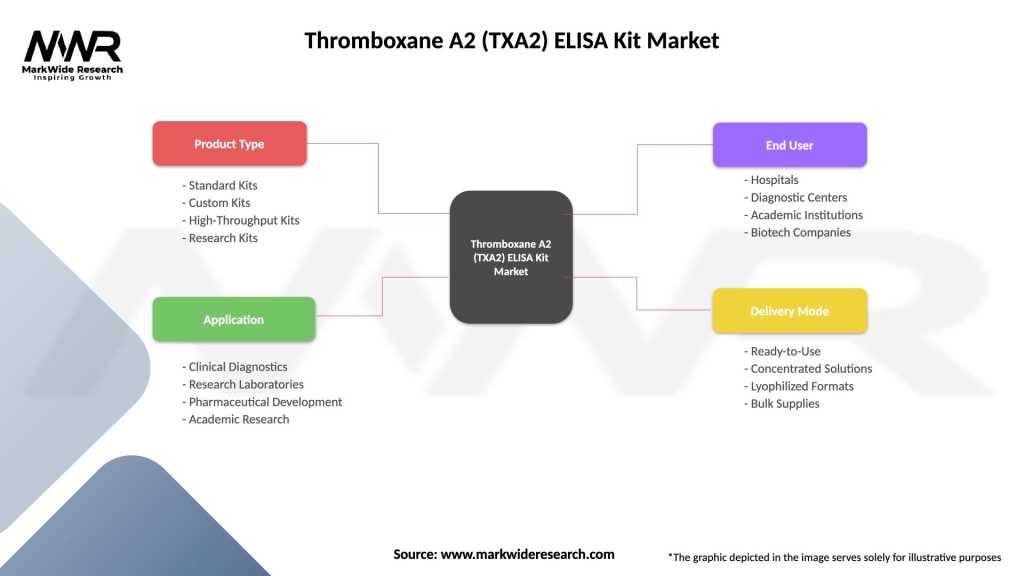
Segmentation
The TXA2 ELISA Kit Market can be segmented based on several factors:
Category-wise Insights
Different categories within the TXA2 ELISA Kit Market offer various benefits and applications:
Key Benefits for Industry Participants and Stakeholders
The TXA2 ELISA Kit Market offers several benefits for industry participants and stakeholders:
SWOT Analysis:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has had a multifaceted impact on the TXA2 ELISA kit market. While the pandemic has diverted resources and attention to Covid-19 testing and research, it has also underscored the importance of cardiovascular health and the need for robust diagnostic tools for cardiovascular diseases. The pandemic has accelerated digital transformation initiatives, telehealth adoption, and remote monitoring solutions, driving demand for TXA2 ELISA kits with digital connectivity and point-of-care capabilities. However, supply chain disruptions, laboratory closures, and funding constraints have posed challenges for market players, affecting product availability, distribution channels, and research funding opportunities.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The TXA2 ELISA kit market is poised for continued growth and innovation, driven by advancements in cardiovascular research, personalized medicine, and diagnostic technologies. Key trends shaping the future of the TX A2 ELISA kit market include digitalization, multiplex assay development, AI integration, and biosensor technology advancements. Market players will focus on addressing assay variability, sample interference, and regulatory challenges while capitalizing on opportunities in point-of-care testing, biomarker discovery, companion diagnostics, and emerging applications. Strategic investments in innovation, quality assurance, customer engagement, and market expansion will be critical for sustaining competitiveness and driving market growth.
Conclusion:
The TXA2 ELISA kit market plays a pivotal role in cardiovascular research, clinical diagnostics, and drug development, offering valuable tools for quantifying thromboxane A2 levels accurately. Despite challenges such as assay variability and regulatory hurdles, the market continues to expand driven by rising disease burden, technological advancements, and clinical diagnostic needs. With ongoing investments in innovation, quality assurance, and market expansion, the future outlook for the TXA2 ELISA kit market remains promising, with opportunities for addressing unmet medical needs, improving patient outcomes, and advancing cardiovascular healthcare globally.
What is Thromboxane A2 (TXA2) ELISA Kit?
Thromboxane A2 (TXA2) ELISA Kit is a laboratory tool used to measure the levels of thromboxane A2, a potent vasoconstrictor and platelet aggregator, in biological samples. It is commonly utilized in research related to cardiovascular diseases, inflammation, and platelet function.
What are the key players in the Thromboxane A2 (TXA2) ELISA Kit Market?
Key players in the Thromboxane A2 (TXA2) ELISA Kit Market include companies like Enzo Life Sciences, R&D Systems, and Abcam, which are known for their contributions to immunoassay technologies and biochemical research, among others.
What are the growth factors driving the Thromboxane A2 (TXA2) ELISA Kit Market?
The growth of the Thromboxane A2 (TXA2) ELISA Kit Market is driven by the increasing prevalence of cardiovascular diseases, rising research activities in platelet biology, and advancements in diagnostic technologies. Additionally, the growing focus on personalized medicine is also contributing to market expansion.
What challenges does the Thromboxane A2 (TXA2) ELISA Kit Market face?
The Thromboxane A2 (TXA2) ELISA Kit Market faces challenges such as the high cost of advanced kits, the need for skilled personnel to conduct assays, and potential regulatory hurdles in different regions. These factors can limit accessibility and adoption in some laboratories.
What opportunities exist in the Thromboxane A2 (TXA2) ELISA Kit Market?
Opportunities in the Thromboxane A2 (TXA2) ELISA Kit Market include the development of more cost-effective and user-friendly kits, expansion into emerging markets, and the integration of these kits into routine clinical diagnostics. Additionally, collaborations between research institutions and manufacturers can enhance innovation.
What trends are shaping the Thromboxane A2 (TXA2) ELISA Kit Market?
Trends in the Thromboxane A2 (TXA2) ELISA Kit Market include the increasing adoption of high-throughput screening methods, advancements in assay sensitivity and specificity, and the growing interest in biomarker discovery for various diseases. These trends are driving innovation and improving research outcomes.
Thromboxane A2 (TXA2) ELISA Kit Market
| Segmentation Details | Description |
|---|---|
| Product Type | Standard Kits, Custom Kits, High-Throughput Kits, Research Kits |
| Application | Clinical Diagnostics, Research Laboratories, Pharmaceutical Development, Academic Research |
| End User | Hospitals, Diagnostic Centers, Academic Institutions, Biotech Companies |
| Delivery Mode | Ready-to-Use, Concentrated Solutions, Lyophilized Formats, Bulk Supplies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Thromboxane A2 (TXA2) ELISA Kit Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at