444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Synthetic Blood Substitutes market is experiencing significant growth due to the rising demand for blood transfusion alternatives and advancements in medical technology. Synthetic blood substitutes, also known as artificial blood, are engineered products that can mimic the oxygen-carrying capacity of natural blood. These substitutes play a crucial role in situations where the availability of donated blood is limited or when transfusion-related risks are a concern. The market for synthetic blood substitutes offers promising opportunities for innovation and improved patient outcomes in various medical settings.
Meaning
Synthetic blood substitutes refer to artificial products that can serve as temporary alternatives to natural blood in medical procedures. These substitutes are designed to replicate the oxygen-carrying function of red blood cells and can be used in situations where whole blood transfusions are not available or contraindicated. Synthetic blood substitutes are typically composed of oxygen-carrying molecules or hemoglobin-based solutions that can restore and maintain tissue oxygenation. These products aim to address the critical need for safe and effective blood transfusion alternatives.
Executive Summary
The Synthetic Blood Substitutes market is witnessing significant growth as a result of technological advancements, increasing demand for blood transfusion alternatives, and the need for improved patient outcomes in emergency and surgical settings. The market is driven by factors such as the rising prevalence of blood-related disorders, the growing number of surgeries and trauma cases, and the limitations associated with the availability and storage of donated blood. However, the market also faces challenges such as regulatory concerns, safety issues, and the complex nature of developing reliable and efficacious synthetic blood substitutes. Despite these obstacles, the market presents attractive opportunities for research and development efforts to enhance patient care and address unmet medical needs.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
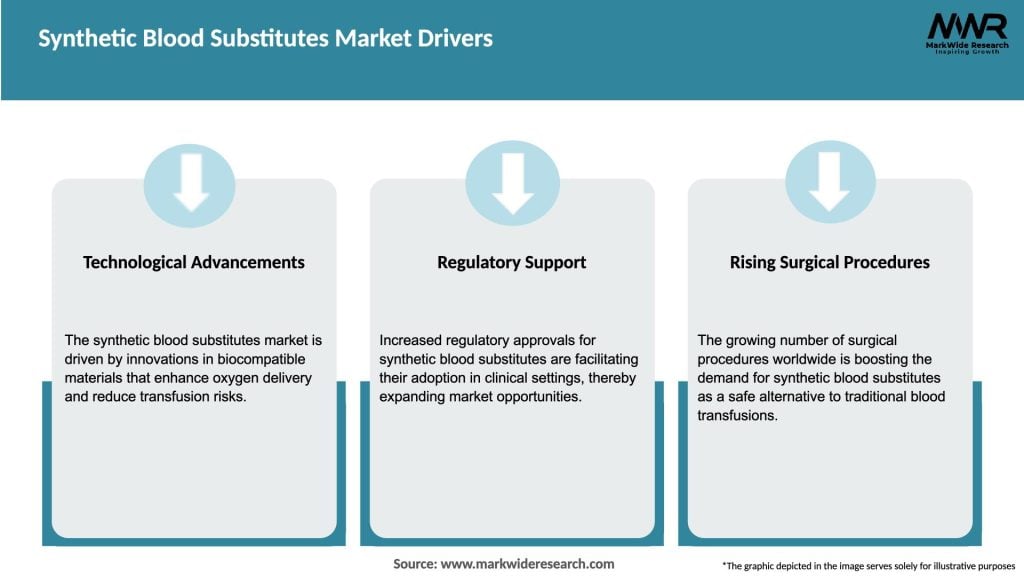
Key Market Insights
Market Drivers
Several factors are driving the growth of the Synthetic Blood Substitutes market:
Market Restraints
Despite the favorable market conditions, several challenges hinder the growth of the Synthetic Blood Substitutes market:
Market Opportunities
The Synthetic Blood Substitutes market presents several opportunities for industry participants:
Market Dynamics
The Synthetic Blood Substitutes market is dynamic and influenced by various factors. These include technological advancements, regulatory landscapes, changing healthcare practices, public awareness, and global health emergencies. Continuous monitoring of market dynamics is crucial for stakeholders to identify trends, adapt strategies, and capitalize on emerging opportunities.
Regional Analysis
The Synthetic Blood Substitutes market exhibits regional variations due to differences in healthcare infrastructure, disease burden, regulatory frameworks, and market maturity. The key regions analyzed in this market are:
Competitive Landscape
Leading Companies in the Synthetic Blood Substitutes Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
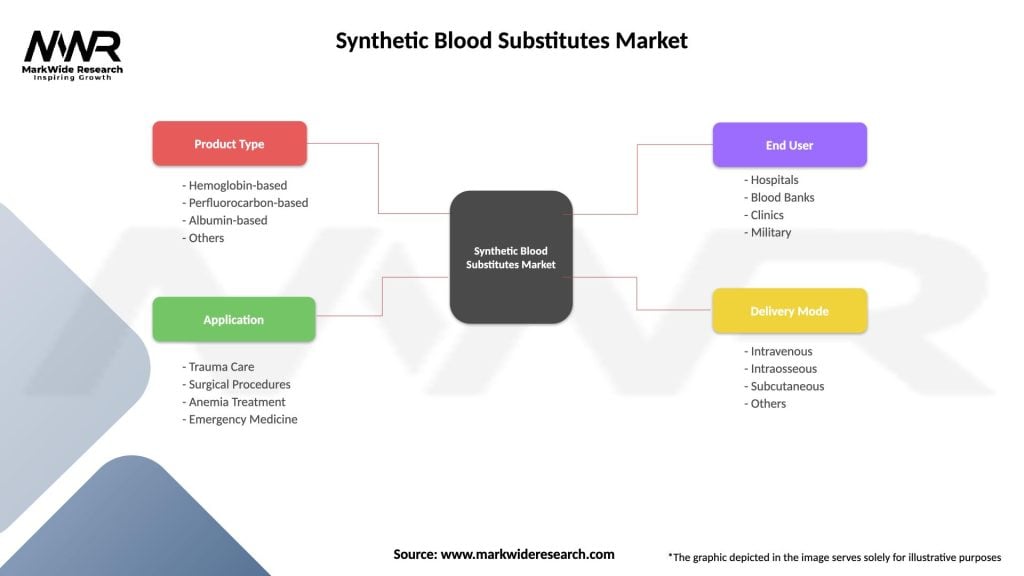
Segmentation
The Synthetic Blood Substitutes market can be segmented based on various factors, including product type, application, end-user, and region. Common segmentation parameters include:
These segmentation parameters help in understanding market dynamics, target specific applications, and tailor strategies to meet the needs of different end-users.
Category-wise Insights
The Synthetic Blood Substitutes market can be further analyzed based on different categories, providing valuable insights into specific aspects of the market. Some category-wise insights include:
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the Synthetic Blood Substitutes market can benefit in several ways:
SWOT Analysis
A SWOT analysis provides an overview of the market’s strengths, weaknesses, opportunities, and threats:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
The Synthetic Blood Substitutes market is influenced by several key trends:
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the healthcare industry, including the Synthetic Blood Substitutes market. Some key impacts include:
Key Industry Developments
The Synthetic Blood Substitutes market has witnessed several notable industry developments:
Analyst Suggestions
Based on market trends and dynamics, analysts provide the following suggestions:
Future Outlook
The future of the Synthetic Blood Substitutes market is promising, with continued growth expected. Advancements in bioengineering, formulation optimization, and manufacturing techniques will drive product improvements. Collaborations, research initiatives, and regulatory adaptations will further streamline market entry and foster innovation. The increasing focus on patient safety, personalized medicine approaches, and the need for alternative transfusion strategies will shape the market’s future landscape.
Conclusion
The Synthetic Blood Substitutes market is witnessing significant growth due to the increasing demand for blood transfusion alternatives and advancements in medical technology. Despite regulatory challenges and safety concerns, the market offers opportunities for innovation and improved patient outcomes. Continued research and development efforts, collaborations, and market education initiatives will drive the growth of the market, paving the way for safer, more effective, and personalized synthetic blood substitutes that cater to the evolving needs of healthcare providers and patients.
What is Synthetic Blood Substitutes?
Synthetic blood substitutes are artificial products designed to mimic the functions of human blood, primarily for use in medical applications such as trauma care, surgery, and blood transfusions. They aim to provide oxygen transport and volume expansion without the risks associated with human blood products.
What are the key players in the Synthetic Blood Substitutes Market?
Key players in the Synthetic Blood Substitutes Market include companies like Hemarina, which focuses on marine hemoglobin-based products, and Sangart, known for its hemoglobin-based oxygen carriers. Other notable companies include Oxycyte and Biopure Corporation, among others.
What are the growth factors driving the Synthetic Blood Substitutes Market?
The growth of the Synthetic Blood Substitutes Market is driven by factors such as the increasing demand for blood transfusions, advancements in biotechnology, and the need for alternatives to human blood products due to safety concerns. Additionally, the rising prevalence of trauma cases and surgical procedures contributes to market expansion.
What challenges does the Synthetic Blood Substitutes Market face?
The Synthetic Blood Substitutes Market faces challenges including regulatory hurdles, the complexity of developing safe and effective substitutes, and public acceptance of synthetic products. Additionally, competition from traditional blood donation systems poses a significant challenge.
What opportunities exist in the Synthetic Blood Substitutes Market?
Opportunities in the Synthetic Blood Substitutes Market include the potential for innovation in product development, particularly in creating more effective oxygen carriers. There is also a growing interest in using synthetic substitutes in emergency medicine and military applications, which could expand market reach.
What trends are shaping the Synthetic Blood Substitutes Market?
Trends in the Synthetic Blood Substitutes Market include increasing research and development efforts focused on improving the efficacy and safety of synthetic products. Additionally, there is a trend towards collaboration between biotech firms and research institutions to accelerate innovation in blood substitute technologies.
Synthetic Blood Substitutes Market
| Segmentation Details | Description |
|---|---|
| Product Type | Hemoglobin-based, Perfluorocarbon-based, Albumin-based, Others |
| Application | Trauma Care, Surgical Procedures, Anemia Treatment, Emergency Medicine |
| End User | Hospitals, Blood Banks, Clinics, Military |
| Delivery Mode | Intravenous, Intraosseous, Subcutaneous, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Synthetic Blood Substitutes Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at