444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Surgical Site Infection Control Market is experiencing significant growth due to the increasing incidence of surgical site infections (SSIs) and the need for effective infection control measures in healthcare settings. SSIs are one of the most common healthcare-associated infections, leading to patient morbidity, prolonged hospital stays, and increased healthcare costs. The market is driven by factors such as the rising number of surgical procedures, the growing awareness about infection prevention, and the implementation of stringent regulations and guidelines by healthcare authorities.
Meaning
Surgical site infection control refers to the strategies and interventions implemented to prevent and manage infections that occur at the surgical site. These infections can affect the incision site, deep tissues, or organs involved in the surgical procedure. Surgical site infections are caused by microorganisms introduced during surgery, including bacteria, viruses, and fungi. Effective infection control measures aim to reduce the risk of SSIs by implementing preoperative, intraoperative, and postoperative interventions, such as antimicrobial prophylaxis, proper surgical site preparation, aseptic techniques, and surveillance.
Executive Summary
The Surgical Site Infection Control Market is projected to witness substantial growth in the coming years. The market is driven by factors such as the increasing number of surgical procedures, the rising awareness about the impact of SSIs on patient outcomes, and the implementation of stringent infection control practices. Surgical site infection control measures aim to reduce the incidence of SSIs, improve patient safety, and enhance healthcare quality. The market is witnessing advancements in technology and the development of innovative products to enhance infection prevention and control.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The surgical site infection control market is driven by a combination of healthcare, technological, and regulatory factors. The increasing number of surgical procedures and the growing awareness about healthcare-associated infections fuel market growth. Stringent regulations and guidelines mandate the implementation of infection control practices, driving the demand for effective solutions. Technological advancements in infection control products and interventions further contribute to market expansion. However, challenges such as limited awareness, high costs, and inadequate reimbursement policies pose barriers to market growth. The market is dynamic, with ongoing efforts to develop innovative products, improve surveillance systems, and enhance healthcare professionals’ knowledge and skills in infection prevention and control.
Regional Analysis
The surgical site infection control market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America holds the largest market share due to the advanced healthcare infrastructure, high surgical volume, and stringent infection control regulations. Europe follows closely, driven by the emphasis on patient safety and quality of care. The Asia Pacific region is expected to witness rapid growth due to the increasing surgical volume, improving healthcare infrastructure, and rising awareness about infection control practices. Latin America and the Middle East and Africa offer opportunities for market players, with improving healthcare systems and a growing focus on infection prevention and control.
Competitive Landscape
Leading companies in the Surgical Site Infection Control Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

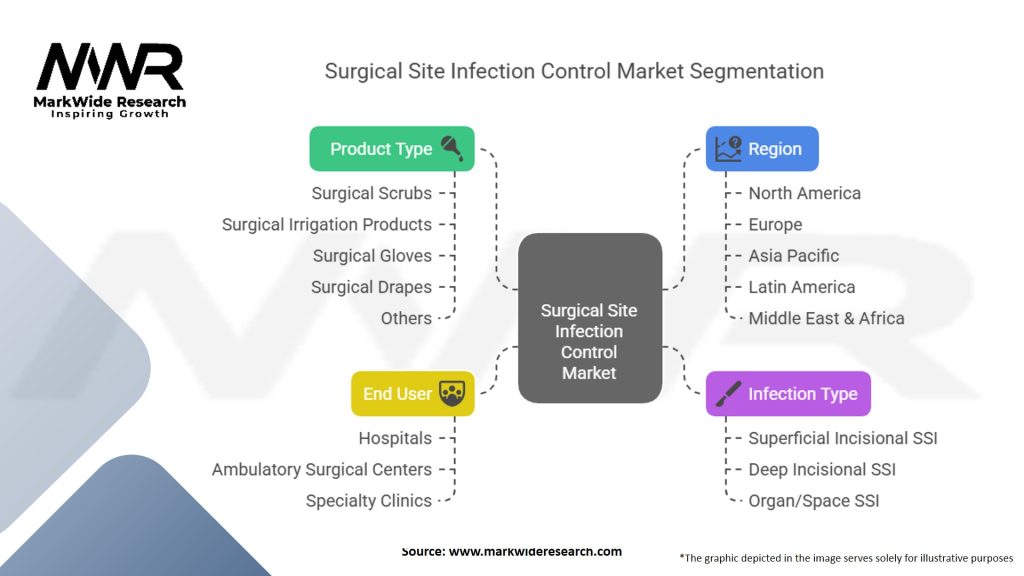
Segmentation
The surgical site infection control market is segmented based on product type, end-user, and region.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the surgical site infection control market. The focus on infection prevention and control has intensified, and healthcare facilities have implemented strict protocols and measures to prevent the transmission of the virus. The pandemic has underscored the importance of maintaining a clean and safe surgical environment. However, the pandemic has also disrupted surgical procedures, leading to a decrease in surgical volume and delayed elective surgeries. As the situation stabilizes and surgical activities resume, the market is expected to rebound and witness steady growth.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Surgical Site Infection Control Market is expected to grow steadily in the coming years. The increasing number of surgical procedures, the growing awareness about the impact of SSIs, and the implementation of stringent infection control practices drive market expansion. Technological advancements, collaborations, and the integration of infection control measures with surgical equipment are key trends in the market. Companies that invest in research and development, prioritize education and training, and foster collaboration are likely to thrive in the evolving landscape of surgical site infection control.
Conclusion
The Surgical Site Infection Control Market offers significant growth opportunities driven by the increasing need for effective infection control measures in surgical settings. SSIs pose a significant risk to patient safety and healthcare quality, making infection prevention and control crucial. The market is driven by the rising number of surgical procedures, growing awareness about healthcare-associated infections, and stringent regulations. Companies that invest in research and development, promote education and training, and collaborate with healthcare facilities and regulatory bodies are well-positioned to succeed in the dynamic field of surgical site infection control.
What is Surgical Site Infection Control?
Surgical Site Infection Control refers to the practices and protocols implemented to prevent infections that occur at the site of a surgical procedure. This includes sterilization techniques, proper wound care, and the use of prophylactic antibiotics.
What are the key companies in the Surgical Site Infection Control Market?
Key companies in the Surgical Site Infection Control Market include Medtronic, Johnson & Johnson, and Becton Dickinson, among others.
What are the main drivers of growth in the Surgical Site Infection Control Market?
The main drivers of growth in the Surgical Site Infection Control Market include the increasing number of surgical procedures, rising awareness about infection prevention, and advancements in surgical technologies.
What challenges does the Surgical Site Infection Control Market face?
Challenges in the Surgical Site Infection Control Market include the rising incidence of antibiotic-resistant infections, variability in compliance with infection control protocols, and the high costs associated with advanced infection control technologies.
What opportunities exist in the Surgical Site Infection Control Market?
Opportunities in the Surgical Site Infection Control Market include the development of innovative infection control products, increasing investments in healthcare infrastructure, and the growing emphasis on patient safety and quality of care.
What trends are shaping the Surgical Site Infection Control Market?
Trends shaping the Surgical Site Infection Control Market include the integration of digital health technologies, the use of antimicrobial coatings on surgical instruments, and a focus on personalized medicine to enhance infection prevention strategies.
Surgical Site Infection Control Market
| Segmentation Details | Description |
|---|---|
| Product Type | Surgical Scrubs, Surgical Irrigation Products, Surgical Gloves, Surgical Drapes, Others |
| Infection Type | Superficial Incisional SSI, Deep Incisional SSI, Organ/Space SSI |
| End User | Hospitals, Ambulatory Surgical Centers, Specialty Clinics |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Surgical Site Infection Control Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at