444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Stable Cell Line Development Market is a pivotal segment within the biotechnology and pharmaceutical industries. Stable cell lines serve as crucial tools for the production of therapeutic proteins, monoclonal antibodies, and vaccines. This comprehensive analysis explores the intricacies of the Stable Cell Line Development Market, covering its meaning, executive summary, key market insights, drivers, restraints, opportunities, dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, benefits for industry participants, SWOT analysis, key trends, the impact of Covid-19, industry developments, analyst suggestions, future outlook, and a conclusive summary.
Meaning
Stable cell line development involves the creation of genetically modified cell lines that can consistently produce specific proteins or biopharmaceutical products. These cell lines are essential for the production of drugs, vaccines, and therapeutic proteins in biotechnology and pharmaceutical industries.
Executive Summary
The Stable Cell Line Development Market is experiencing significant growth, driven by the increasing demand for biopharmaceuticals, including monoclonal antibodies and gene therapies. Stable cell lines are essential for the production of these complex biologics. However, the market faces challenges related to cell line stability, regulatory compliance, and the need for advanced technologies to streamline the development process.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
1. Growing Biopharmaceutical Market
The increasing demand for biopharmaceuticals drives the need for stable cell lines for production.
2. Monoclonal Antibodies
Monoclonal antibodies play a significant role in the treatment of various diseases, boosting the demand for stable cell lines.
3. Gene Therapies
Advancements in gene therapies require stable cell lines for the production of viral vectors and therapeutic proteins.
4. Regulatory Approvals
Regulatory approvals for biopharmaceuticals necessitate compliance with cell line development and production standards.
Market Restraints
1. Cell Line Stability
Maintaining cell line stability over time is a challenge, impacting consistent production.
2. Regulatory Compliance
Meeting regulatory requirements, including those related to quality control and validation, can be complex and resource-intensive.
3. Technological Advancements
Ongoing innovation is necessary to streamline the stable cell line development process.
Market Opportunities
1. Advanced Technologies
The development of advanced technologies, such as CRISPR-Cas9 gene editing, enhances the efficiency of stable cell line development.
2. Personalized Medicine
The trend toward personalized medicine creates opportunities for the development of customized stable cell lines for individualized therapies.
3. Vaccine Production
The demand for vaccines, including those for emerging infectious diseases, presents growth opportunities in stable cell line development.
Market Dynamics
The Stable Cell Line Development Market is characterized by dynamic shifts influenced by technological advancements, regulatory changes, and the evolving biopharmaceutical landscape. Understanding these dynamics is crucial for industry participants to stay competitive and seize emerging opportunities.
Regional Analysis
The market for Stable Cell Line Development varies by region due to differences in biopharmaceutical production, research activities, and regulatory environments. A regional analysis provides insights into the market’s performance in different parts of the world.
North America
North America, particularly the United States, is a leading market for Stable Cell Line Development, driven by its robust biopharmaceutical industry and research activities.
Europe
Europe is a significant market for stable cell line development, with countries like the United Kingdom, Germany, and Switzerland at the forefront of biotechnology and pharmaceutical research.
Asia-Pacific
The Asia-Pacific region, led by China, India, and South Korea, is experiencing rapid growth in the Stable Cell Line Development Market due to the expansion of biopharmaceutical manufacturing and research capabilities.
Latin America
Latin America presents growth opportunities driven by increasing biopharmaceutical production and research investments.
Middle East and Africa
The Middle East and Africa are emerging markets for stable cell line development, with investments in biopharmaceutical infrastructure and research facilities.
Competitive Landscape
Leading Companies in the Stable Cell Line Development Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
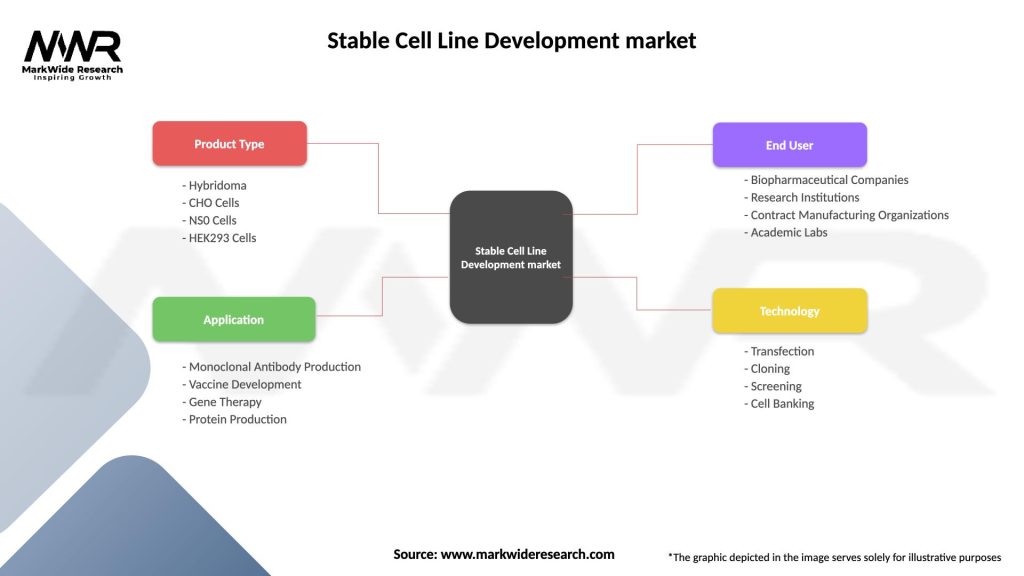
The Stable Cell Line Development Market can be segmented based on various factors, including cell type, application, technology, and region.
By Cell Type
By Application
By Technology
By Region
Category-wise Insights
Mammalian Cells vs. Bacterial Cells
The choice between mammalian and bacterial cells depends on the specific biopharmaceutical product, production scale, and regulatory requirements.
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths
Weaknesses
Opportunities
Threats
Market Key Trends
1. Advanced Gene Editing
The adoption of advanced gene editing technologies such as CRISPR-Cas9 for precise modifications.
2. Personalized Medicine
The trend toward personalized medicine creating demand for customized stable cell lines.
3. Vaccine Development
The growing demand for vaccines, including those for emerging infectious diseases, driving the need for stable cell lines.
Covid-19 Impact
The Covid-19 pandemic accelerated research and development efforts in biopharmaceuticals, including vaccines and therapeutic antibodies. Stable cell lines played a crucial role in vaccine production and monoclonal antibody therapies for Covid-19.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the Stable Cell Line Development Market is promising, with continued growth expected. As the demand for biopharmaceuticals, gene therapies, and vaccines continues to rise, the need for stable cell lines will remain robust. Industry participants must focus on advanced technologies, customization, and regulatory compliance to thrive in this dynamic and essential market.
Conclusion
In conclusion, the Stable Cell Line Development Market plays a pivotal role in the biotechnology and pharmaceutical industries, facilitating the production of biopharmaceuticals, gene therapies, and vaccines. Despite challenges related to cell line stability and regulatory compliance, the market offers significant growth opportunities driven by the increasing demand for innovative therapies. Industry participants must adapt to evolving trends, invest in advanced technologies, and explore customization to meet the diverse needs of the biopharmaceutical and healthcare sectors.
What is Stable Cell Line Development?
Stable Cell Line Development refers to the process of creating cell lines that stably express a specific gene or protein of interest. These cell lines are essential for various applications, including drug development, protein production, and gene therapy research.
What are the key players in the Stable Cell Line Development market?
Key players in the Stable Cell Line Development market include Thermo Fisher Scientific, Merck KGaA, and Lonza Group, among others. These companies are known for their innovative technologies and comprehensive services in cell line development.
What are the main drivers of the Stable Cell Line Development market?
The main drivers of the Stable Cell Line Development market include the increasing demand for biopharmaceuticals, advancements in genetic engineering technologies, and the growing focus on personalized medicine. These factors are propelling the need for efficient and reliable cell line development processes.
What challenges does the Stable Cell Line Development market face?
The Stable Cell Line Development market faces challenges such as the high costs associated with development and maintenance of stable cell lines, regulatory hurdles, and the complexity of ensuring consistent performance across different cell lines. These challenges can hinder the speed of research and development.
What opportunities exist in the Stable Cell Line Development market?
Opportunities in the Stable Cell Line Development market include the rising demand for monoclonal antibodies, the expansion of cell-based therapies, and the increasing investment in biotechnology research. These trends are likely to drive innovation and growth in the sector.
What trends are shaping the Stable Cell Line Development market?
Trends shaping the Stable Cell Line Development market include the integration of automation and high-throughput screening technologies, the use of CRISPR/Cas9 for gene editing, and the development of next-generation cell lines. These innovations are enhancing the efficiency and effectiveness of cell line development.
Stable Cell Line Development market
| Segmentation Details | Description |
|---|---|
| Product Type | Hybridoma, CHO Cells, NS0 Cells, HEK293 Cells |
| Application | Monoclonal Antibody Production, Vaccine Development, Gene Therapy, Protein Production |
| End User | Biopharmaceutical Companies, Research Institutions, Contract Manufacturing Organizations, Academic Labs |
| Technology | Transfection, Cloning, Screening, Cell Banking |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Stable Cell Line Development Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at