444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The spinal fusion devices market is experiencing significant growth and is poised to expand further in the coming years. Spinal fusion is a surgical procedure used to join two or more vertebrae in the spine, providing stability and reducing pain. The demand for spinal fusion devices has increased due to the rising prevalence of spinal disorders, such as degenerative disc disease, spinal stenosis, and spondylolisthesis, among others. These conditions often cause chronic pain and can severely impact an individual’s quality of life. As a result, the need for effective treatment options, including spinal fusion surgeries, has grown.
Spinal fusion devices refer to the instruments, implants, and technologies used during spinal fusion procedures. These devices aid in stabilizing the spine, promoting bone growth between fused vertebrae, and restoring proper alignment. The main objective of spinal fusion is to eliminate or reduce pain, restore spinal function, and prevent further damage. The market for spinal fusion devices encompasses a wide range of products, including spinal implants, bone grafts, spinal fixation systems, and other surgical instruments necessary for the procedure.
Executive Summary
The global spinal fusion devices market has witnessed substantial growth in recent years, driven by the increasing prevalence of spinal disorders and advancements in surgical techniques. The market is characterized by intense competition among key players, who are constantly striving to introduce innovative products and technologies. With a focus on improving patient outcomes and reducing post-operative complications, manufacturers are investing heavily in research and development activities.
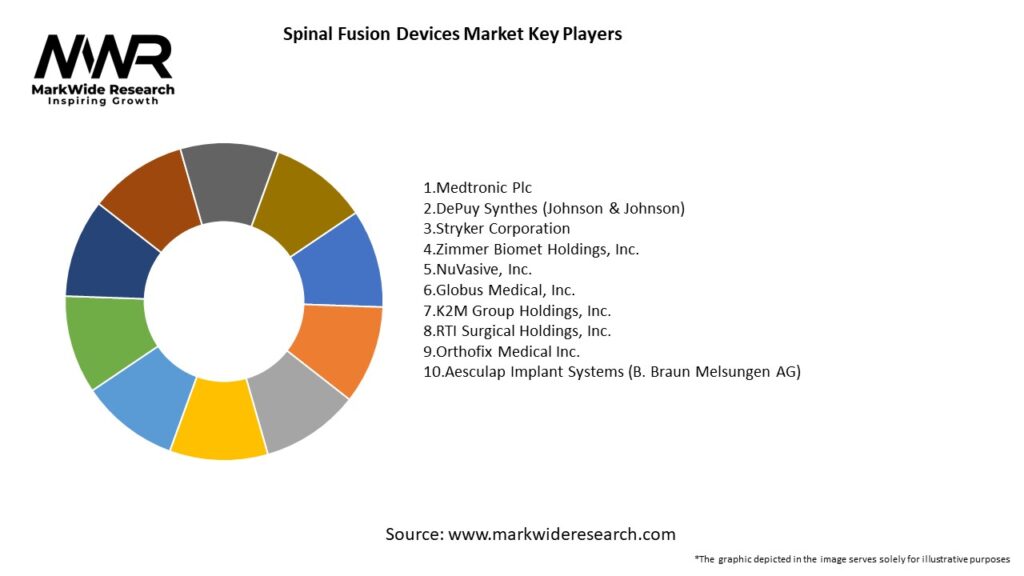
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
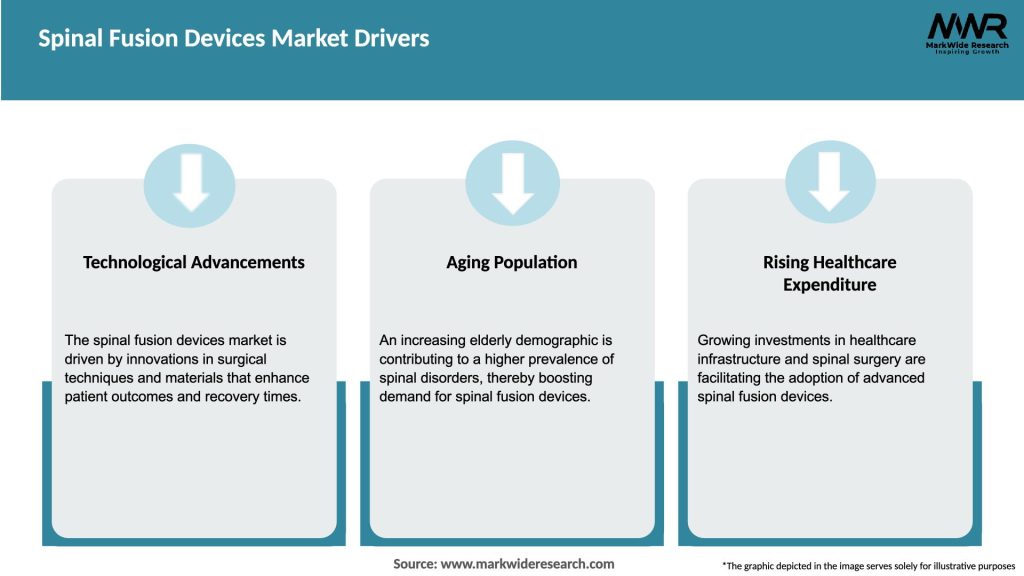
Several factors are driving the growth of the spinal fusion devices market:
Market Restraints
Despite the positive market outlook, certain factors may restrain the growth of the spinal fusion devices market:
Market Opportunities
The spinal fusion devices market presents several opportunities for growth and development:
Market Dynamics
The spinal fusion devices market is highly dynamic, influenced by various factors such as technological advancements, changing demographics, market competition, and regulatory landscape. Manufacturers need to stay abreast of these dynamics to effectively navigate the market and ensure sustainable growth.
Technological advancements in spinal fusion devices continue to shape the market. Minimally invasive procedures, navigation systems, 3D-printed implants, and biologics have transformed the landscape of spinal surgeries. These innovations aim to improve patient outcomes, reduce complications, and enhance the overall surgical experience.
Demographic changes, such as an aging population, contribute to the growth of the market. Older individuals are more prone to spinal disorders, creating a larger patient pool for spinal fusion surgeries. Additionally, sedentary lifestyles and unhealthy habits further contribute to the prevalence of spinal conditions, driving the demand for treatment options.
Market competition is intense, with several key players vying for market share. Companies are investing in research and development activities to introduce innovative products and gain a competitive edge. Mergers, acquisitions, and strategic collaborations are common strategies employed by market players to expand their product portfolios and enhance their market presence.
The regulatory landscape significantly impacts the market dynamics. Compliance with regulatory requirements, such as obtaining necessary approvals and certifications, is crucial for market entry and product commercialization. Stringent regulations ensure patient safety but can pose challenges for new entrants and delay product launches.
Furthermore, economic factors, reimbursement policies, and healthcare infrastructure influence market dynamics. Economic stability, favorable reimbursement systems, and access to quality healthcare facilities play a vital role in driving the adoption of spinal fusion devices.
Regional Analysis
The spinal fusion devices market can be analyzed on a regional basis to gain insights into geographical trends and opportunities. The market is categorized into several regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
North America: North America dominates the global spinal fusion devices market due to the presence of well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced technologies. The region also benefits from strong reimbursement systems and a large patient pool.
Europe: Europe holds a significant market share and is driven by factors such as a growing aging population, increasing prevalence of spinal disorders, and advancements in surgical techniques. Countries such as Germany, the United Kingdom, and France contribute significantly to the market growth.
Asia-Pacific: The Asia-Pacific region is expected to witness rapid market growth due to factors such as a large patient population, improving healthcare infrastructure, and increasing awareness about spinal disorders. Countries like China, India, and Japan present lucrative opportunities for market players.
Latin America: Latin America is a growing market with improving healthcare access and increasing investments in healthcare infrastructure. The region’s rising prevalence of spinal disorders and favorable government initiatives contribute to market growth.
Middle East and Africa: The Middle East and Africa region offer opportunities for market expansion, primarily driven by increasing healthcare expenditure, infrastructure development, and the rising prevalence of spinal disorders. The market growth in this region can be attributed to countries such as Saudi Arabia, the United Arab Emirates, and South Africa.
Understanding the regional dynamics, cultural variations, and regulatory frameworks is essential for market players to devise region-specific strategies and effectively cater to the needs of the target population.
Competitive Landscape
Leading Companies in the Spinal Fusion Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
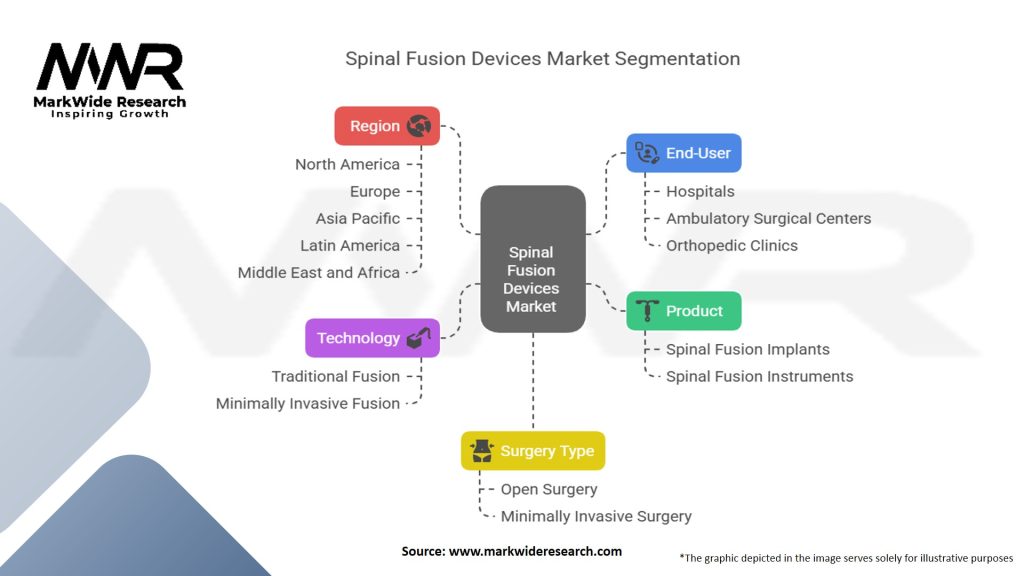
Segmentation
The spinal fusion devices market can be segmented based on various factors, including product type, surgery type, end-user, and region.
By Product Type:
By Surgery Type:
By End-User:
The segmentation allows for a deeper analysis of the market, providing insights into specific product categories, surgical procedures, and end-users. Understanding these segments helps manufacturers tailor their strategies and offerings to meet the unique requirements of different customer groups.
Category-wise Insights
The spinal fusion devices market encompasses various categories that contribute to its overall growth and development. Let’s explore the insights and trends within each category:
Spinal Implants: Spinal implants, including rods, screws, plates, and cages, are crucial components of spinal fusion surgeries. The demand for spinal implants is driven by factors such as increasing prevalence of spinal disorders, advancements in implant materials, and the need for improved surgical outcomes. Companies invest in research and development to introduce innovative implant designs, surface technologies, and biocompatible materials that enhance fusion rates, reduce complications, and provide long-term stability.
Spinal Fixation Systems: Spinal fixation systems play a vital role in providing stability and support during spinal fusion surgeries. These systems include pedicle screw-based systems, facet fixation systems, and hybrid systems that combine multiple fixation techniques. Advancements in spinal fixation systems aim to offer improved biomechanical stability, reduced surgical invasiveness, and better patient outcomes. Manufacturers focus on developing systems that allow for customization, easy implantation, and enhanced surgical accuracy.
Bone Grafts: Bone grafts are used to facilitate bone fusion in spinal surgeries. Autografts, allografts, and synthetic grafts are commonly used in spinal fusion procedures. The choice of bone graft material depends on factors such as fusion rates, availability, surgeon preference, and patient-specific considerations. Manufacturers invest in research to develop graft materials with osteoinductive, osteoconductive, and osteogenic properties that promote bone healing and fusion.
Others (Surgical Instruments, Bone Cement, etc.): In addition to implants and fixation systems, the spinal fusion devices market encompasses various other products. Surgical instruments, including retractors, drills, and curettes, play a crucial role in facilitating surgical procedures. Bone cement is used to enhance stability and provide additional support during spinal fusion surgeries. Manufacturers continue to innovate in these areas to develop specialized instruments and cement formulations that improve surgical accuracy and patient outcomes.
Understanding the trends and advancements within each category allows manufacturers to focus their research and development efforts, introduce differentiated products, and address specific challenges associated with each product category.
Key Benefits for Industry Participants and Stakeholders
The spinal fusion devices market offers several benefits for industry participants and stakeholders, including:
Understanding the benefits associated with the spinal fusion devices market allows industry participants and stakeholders to align their strategies, prioritize investment areas, and create value for all involved parties.
SWOT Analysis
A SWOT analysis helps evaluate the strengths, weaknesses, opportunities, and threats associated with the spinal fusion devices market:
Strengths:
Weaknesses:
Opportunities:
Threats:
Understanding the SWOT analysis enables industry participants to leverage their strengths, address weaknesses, capitalize on opportunities, and mitigate potential threats. This analysis serves as a valuable tool for strategic decision-making and positioning in the market.
Market Key Trends
The spinal fusion devices market is shaped by several key trends that influence its growth and direction. Recognizing these trends allows industry participants to adapt their strategies and offerings accordingly:
Understanding these key trends enables industry participants to align their strategies with the evolving needs of the market, cater to changing surgeon and patient preferences, and deliver innovative solutions that drive positive outcomes.
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the healthcare industry, including the spinal fusion devices market. The pandemic led to disruptions in healthcare services, elective surgeries, and supply chains. Here are some key considerations regarding the impact of Covid-19 on the market:
As the healthcare sector adapts to the post-pandemic landscape, industry participants need to remain agile, adapt to new paradigms of care, and align their strategies with the evolving needs of the market and healthcare systems.
Key Industry Developments
The spinal fusion devices market has witnessed several key industry developments in recent years. These developments have shaped the market landscape and influenced the direction of research, product innovation, and market competition. Some notable developments include:
These industry developments highlight the continuous evolution and innovation within the spinal fusion devices market. Industry participants need to stay abreast of these developments, invest in research and development, and adapt their strategies to maintain a competitive edge.
Analyst Suggestions
Based on the analysis of the spinal fusion devices market, industry analysts make the following suggestions for market participants:
Future Outlook
The future of the spinal fusion devices market holds promising opportunities for growth, driven by technological advancements, increasing prevalence of spinal disorders, and a growing focus on personalized healthcare. Here are key aspects shaping the future outlook:
Conclusion
The spinal fusion devices market presents lucrative opportunities for industry participants, driven by the increasing prevalence of spinal disorders, technological advancements, and the focus on personalized healthcare. To succeed in this competitive landscape, companies should prioritize innovation, embrace minimally invasive approaches, collaborate with surgeons and research institutions, and leverage digital solutions. By staying abreast of regulatory changes, expanding into emerging markets, and prioritizing patient education and engagement, industry participants can position themselves for growth and deliver positive patient outcomes. The future outlook of the spinal fusion devices market is promising, with continued advancements and a patient-centric approach shaping the trajectory of the industry.
What are spinal fusion devices?
Spinal fusion devices are medical instruments used to join two or more vertebrae in the spine, promoting healing and stability. They are commonly used in procedures to treat conditions such as degenerative disc disease, spinal stenosis, and spondylolisthesis.
Who are the key players in the spinal fusion devices market?
Key players in the spinal fusion devices market include Medtronic, DePuy Synthes, Stryker, and NuVasive, among others. These companies are known for their innovative products and extensive research in spinal surgery technologies.
What are the main drivers of growth in the spinal fusion devices market?
The main drivers of growth in the spinal fusion devices market include the increasing prevalence of spinal disorders, advancements in surgical techniques, and the rising aging population. Additionally, the demand for minimally invasive procedures is also contributing to market expansion.
What challenges does the spinal fusion devices market face?
The spinal fusion devices market faces challenges such as high costs associated with surgical procedures and potential complications from surgeries. Furthermore, the availability of alternative treatments may also hinder market growth.
What opportunities exist in the spinal fusion devices market?
Opportunities in the spinal fusion devices market include the development of new technologies such as bioactive materials and robotic-assisted surgery. Additionally, expanding into emerging markets presents significant growth potential for manufacturers.
What trends are shaping the spinal fusion devices market?
Trends shaping the spinal fusion devices market include the increasing adoption of minimally invasive surgical techniques and the integration of advanced imaging technologies. There is also a growing focus on patient-specific implants and personalized medicine in spinal surgery.
Spinal Fusion Devices Market
| Segmentation | Details |
|---|---|
| Product | Spinal Fusion Implants, Spinal Fusion Instruments |
| Technology | Traditional Fusion, Minimally Invasive Fusion |
| Surgery Type | Open Surgery, Minimally Invasive Surgery |
| End-User | Hospitals, Ambulatory Surgical Centers, Orthopedic Clinics |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Spinal Fusion Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at