444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview:
The Spain Neurothrombectomy Devices market stands at the forefront of medical innovation, offering cutting-edge solutions for the treatment of acute ischemic stroke caused by large vessel occlusion (LVO). Neurothrombectomy devices, including stent retrievers, aspiration catheters, and thromboaspiration systems, play a pivotal role in restoring blood flow to the brain, reducing disability, and improving patient outcomes. With a growing emphasis on early intervention, endovascular therapies, and multidisciplinary stroke care, the Spain Neurothrombectomy Devices market continues to evolve, driven by advancements in technology, clinical evidence, and collaborative efforts across healthcare stakeholders.
Meaning:
The Spain Neurothrombectomy Devices market encompasses a diverse range of medical devices and technologies designed to remove blood clots from cerebral arteries and restore perfusion to ischemic brain tissue in patients experiencing acute ischemic stroke. These devices are deployed via minimally invasive endovascular procedures, guided by advanced imaging techniques, to achieve rapid recanalization of occluded vessels and improve neurological outcomes. Neurothrombectomy represents a paradigm shift in stroke treatment, offering new hope for patients and revolutionizing stroke care pathways.
Executive Summary:
The Spain Neurothrombectomy Devices market is characterized by its dynamic nature, driven by a convergence of technological innovation, clinical research, and healthcare policy reforms aimed at improving stroke care quality and accessibility. As a critical component of the acute stroke care continuum, neurothrombectomy devices offer significant clinical benefits, including higher reperfusion rates, reduced disability, and improved functional independence for stroke survivors. Understanding key market insights, emerging trends, and regulatory considerations is essential for stakeholders to navigate this complex and rapidly evolving landscape effectively.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Spain Neurothrombectomy Devices market operates within a dynamic ecosystem shaped by evolving clinical practices, technological innovations, regulatory frameworks, and socioeconomic factors. Market dynamics such as changing demographics, healthcare reforms, reimbursement policies, and competitive landscapes influence stakeholder behaviors, market strategies, and patient outcomes.
Regional Analysis:
The Spain Neurothrombectomy Devices market exhibits regional variations in stroke incidence, treatment patterns, and healthcare infrastructure across different autonomous communities and provinces. Urban centers, including Madrid, Barcelona, and Valencia, are home to leading academic medical centers, specialized stroke units, and high-volume neurointerventional facilities, driving innovation and setting benchmarks for stroke care quality and accessibility.
Competitive Landscape:
Leading Companies in Spain Neurothrombectomy Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
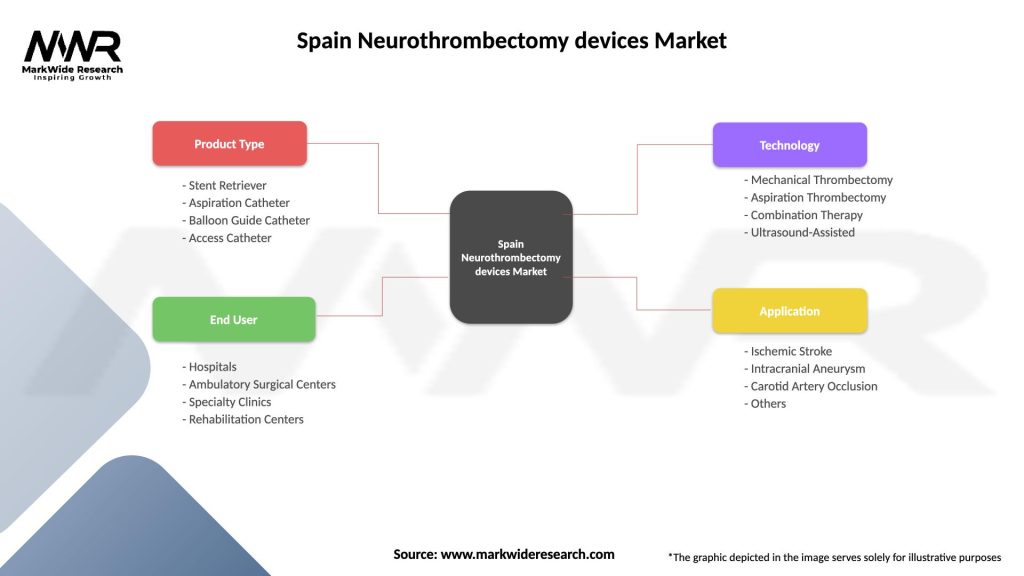
Segmentation:
The Spain Neurothrombectomy Devices market can be segmented based on various criteria, including device type, mechanism of action, patient selection criteria, and procedural technique, to provide a comprehensive understanding of market dynamics, clinical needs, and patient preferences.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
A SWOT analysis of the Spain Neurothrombectomy Devices market reveals key strengths, weaknesses, opportunities, and threats facing industry participants and stakeholders, guiding strategic decision-making, risk management, and market positioning efforts.
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has profound implications for the Spain Neurothrombectomy Devices market, influencing healthcare delivery models, patient behaviors, and resource allocation priorities amidst public health crises, capacity constraints, and economic uncertainties. Key Covid-19 impacts on the market include:
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The Spain Neurothrombectomy Devices market is poised for continued growth and innovation, driven by advancements in device technology, expanding treatment indications, and a growing emphasis on comprehensive stroke care models. While challenges such as treatment disparities, regulatory complexities, and Covid-19-related disruptions persist, proactive measures to address these challenges, foster collaboration, and promote patient-centered care will shape the future of stroke treatment in Spain.
Conclusion:
In conclusion, the Spain Neurothrombectomy Devices market represents a dynamic and transformative domain within the field of acute stroke care, offering innovative solutions to address the unmet clinical needs of patients with LVO strokes. By embracing technological advancements, promoting evidence-based practice, and fostering collaboration across healthcare sectors, industry stakeholders can navigate market dynamics, improve patient outcomes, and contribute to the advancement of stroke care delivery in Spain.
What is Neurothrombectomy devices?
Neurothrombectomy devices are specialized medical instruments used to remove blood clots from the brain’s blood vessels, primarily in patients suffering from ischemic strokes. These devices play a crucial role in restoring blood flow and minimizing brain damage during acute stroke interventions.
What are the key players in the Spain Neurothrombectomy devices Market?
Key players in the Spain Neurothrombectomy devices Market include Medtronic, Stryker, and Penumbra, which are known for their innovative products and technologies in the field of neurovascular interventions. These companies focus on developing advanced devices that enhance patient outcomes and procedural efficiency, among others.
What are the growth factors driving the Spain Neurothrombectomy devices Market?
The Spain Neurothrombectomy devices Market is driven by factors such as the increasing prevalence of stroke cases, advancements in medical technology, and rising awareness about the benefits of timely intervention. Additionally, the growing geriatric population contributes to the demand for these devices.
What challenges does the Spain Neurothrombectomy devices Market face?
Challenges in the Spain Neurothrombectomy devices Market include high costs associated with advanced devices, the need for skilled professionals to operate them, and regulatory hurdles that can delay product approvals. These factors can limit market growth and accessibility.
What opportunities exist in the Spain Neurothrombectomy devices Market?
Opportunities in the Spain Neurothrombectomy devices Market include the potential for technological innovations, such as the development of more efficient and safer devices. Additionally, increasing investments in healthcare infrastructure and rising government initiatives to improve stroke care present significant growth prospects.
What trends are shaping the Spain Neurothrombectomy devices Market?
Trends in the Spain Neurothrombectomy devices Market include the integration of digital technologies, such as AI and machine learning, to enhance procedural outcomes. Furthermore, there is a growing focus on minimally invasive techniques and personalized medicine, which are expected to drive future developments in this field.
Spain Neurothrombectomy devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Stent Retriever, Aspiration Catheter, Balloon Guide Catheter, Access Catheter |
| End User | Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Rehabilitation Centers |
| Technology | Mechanical Thrombectomy, Aspiration Thrombectomy, Combination Therapy, Ultrasound-Assisted |
| Application | Ischemic Stroke, Intracranial Aneurysm, Carotid Artery Occlusion, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Spain Neurothrombectomy Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at