444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The Spain Neonatal and Prenatal Devices Market is experiencing significant growth due to various factors such as advancements in technology, increasing healthcare expenditure, and rising awareness about neonatal and prenatal care. Neonatal and prenatal devices are designed to provide specialized care to newborn babies and expectant mothers, ensuring their well-being and safety throughout the pregnancy and early stages of life. These devices play a crucial role in monitoring, diagnosing, and treating various health conditions related to newborns and pregnant women.
Meaning
Neonatal and prenatal devices refer to a range of medical equipment and instruments specifically designed for the care and treatment of newborns and expectant mothers. These devices aid in the monitoring and management of critical parameters such as heart rate, oxygen saturation, blood pressure, temperature, and other vital signs. They are essential in diagnosing and treating conditions that affect neonates and pregnant women, ensuring their health and safety during and after pregnancy.
Executive Summary
The Spain Neonatal and Prenatal Devices Market is witnessing substantial growth, driven by factors such as the rising prevalence of neonatal and prenatal diseases, increasing government initiatives to improve healthcare infrastructure, and growing investments in research and development activities. The market offers a wide range of devices, including fetal monitors, incubators, phototherapy equipment, respiratory assistance devices, and others. These devices are crucial for effective monitoring and treatment of newborns and expectant mothers, contributing to enhanced healthcare outcomes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
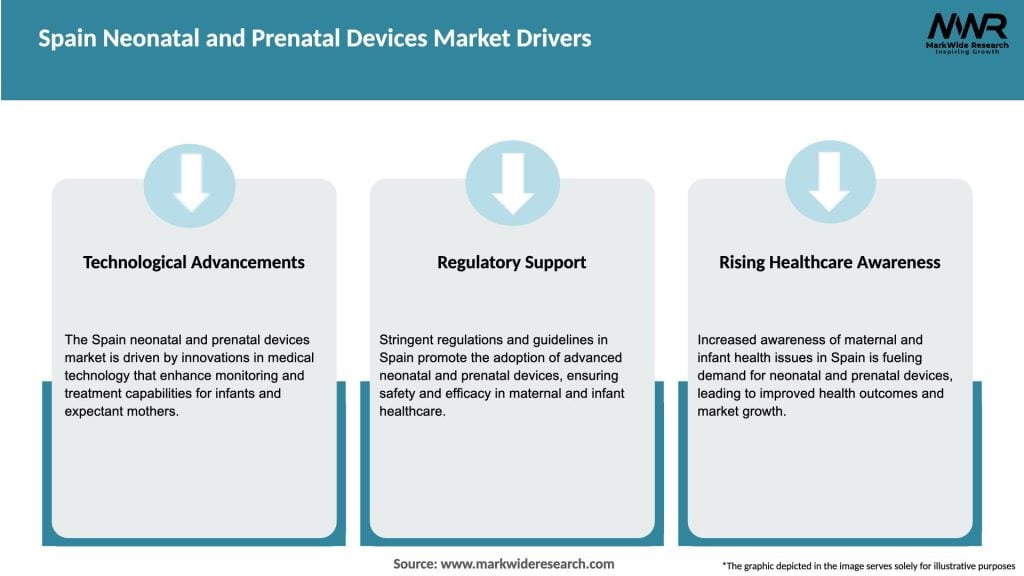
Market Drivers
Market Restraints
Market Opportunities

Market Dynamics
The Spain Neonatal and Prenatal Devices Market is characterized by intense competition among key players striving to enhance their market share. The market is driven by technological advancements, increasing prevalence of neonatal and prenatal diseases, and growing awareness about the importance of early diagnosis and treatment. However, challenges such as high device costs, stringent regulatory requirements, and limited healthcare infrastructure may hinder market growth. Nevertheless, the market presents several opportunities for players to leverage telemedicine, tap into emerging markets, foster product innovation, and engage in collaborative partnerships.
Regional Analysis
The neonatal and prenatal devices market in Spain is experiencing steady growth due to robust healthcare infrastructure, increasing government initiatives, and rising awareness about the importance of neonatal and prenatal care. The country has well-established healthcare facilities and a supportive regulatory environment, which facilitates the adoption and utilization of advanced medical devices. Furthermore, Spain is witnessing a rise in healthcare expenditure and research activities, contributing to the overall development of the neonatal and prenatal devices market.
Competitive Landscape
Leading Companies in Spain Neonatal and Prenatal Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Spain Neonatal and Prenatal Devices Market can be segmented based on device type, end-user, and application. Device types include fetal monitors, incubators, phototherapy equipment, respiratory assistance devices, and others. End-users of these devices include hospitals, clinics, and homecare settings. Applications of neonatal and prenatal devices encompass monitoring, diagnosis, and treatment of various conditions such as preterm birth, respiratory distress syndrome, jaundice, and others.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the Spain Neonatal and Prenatal Devices Market. The healthcare system faced challenges due to the strain on resources and the redirection of attention and resources towards managing the pandemic. Non-essential procedures and appointments were postponed or canceled, impacting the demand for neonatal and prenatal devices. However, the pandemic also highlighted the importance of remote monitoring and telemedicine, leading to increased adoption of such solutions in neonatal and prenatal care.
The pandemic also emphasized the need for infection control measures in healthcare settings. Manufacturers have focused on developing devices with improved sterilization capabilities and antimicrobial properties to minimize the risk of infections. Furthermore, the pandemic accelerated the adoption of digital health technologies, remote patient monitoring, and home-based care, which are expected to continue even after the pandemic.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Spain Neonatal and Prenatal Devices Market is poised for steady growth in the coming years. The market will be driven by technological advancements, increasing prevalence of neonatal and prenatal diseases, and the growing emphasis on early diagnosis and treatment. Telemedicine and remote monitoring solutions are expected to play a crucial role in enhancing accessibility and efficiency of neonatal and prenatal care. The market is likely to witness continuous innovation, product launches, and strategic collaborations among key market players. Overall, the future outlook for the Spain Neonatal and Prenatal Devices Market is optimistic, with opportunities for growth and improvements in healthcare outcomes.
Conclusion
The Spain Neonatal and Prenatal Devices Market is experiencing significant growth, driven by factors such as technological advancements, rising prevalence of neonatal and prenatal diseases, and increasing awareness about the importance of early diagnosis and treatment. The market offers a wide range of devices that cater to the specific needs of newborns and expectant mothers, ensuring their well-being and safety. Although challenges such as high device costs and stringent regulatory requirements exist, opportunities such as the focus on telemedicine, emerging markets, and product innovation offer avenues for market growth. By leveraging these opportunities and addressing industry challenges, market participants can contribute to improved neonatal and prenatal care, enhancing patient outcomes and shaping the future of healthcare.
What is Neonatal and Prenatal Devices?
Neonatal and Prenatal Devices refer to medical equipment and technologies designed for the care of newborns and expectant mothers. These devices include incubators, fetal monitors, and ultrasound machines, which are essential for monitoring and ensuring the health of both mothers and infants.
What are the key players in the Spain Neonatal and Prenatal Devices Market?
Key players in the Spain Neonatal and Prenatal Devices Market include companies like Philips Healthcare, GE Healthcare, and Siemens Healthineers, which provide a range of innovative solutions for maternal and infant care, among others.
What are the growth factors driving the Spain Neonatal and Prenatal Devices Market?
The Spain Neonatal and Prenatal Devices Market is driven by factors such as increasing awareness of maternal and infant health, advancements in medical technology, and a rise in the number of premature births requiring specialized care.
What challenges does the Spain Neonatal and Prenatal Devices Market face?
Challenges in the Spain Neonatal and Prenatal Devices Market include high costs of advanced medical equipment, regulatory hurdles for new device approvals, and the need for continuous training of healthcare professionals to effectively use these technologies.
What opportunities exist in the Spain Neonatal and Prenatal Devices Market?
Opportunities in the Spain Neonatal and Prenatal Devices Market include the development of telemedicine solutions for remote monitoring, increasing investments in healthcare infrastructure, and the potential for innovative product launches tailored to specific maternal and neonatal needs.
What trends are shaping the Spain Neonatal and Prenatal Devices Market?
Trends in the Spain Neonatal and Prenatal Devices Market include the integration of artificial intelligence in monitoring systems, the rise of portable and home-use devices, and a growing emphasis on personalized medicine for maternal and infant care.
Spain Neonatal and Prenatal Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Ultrasound Devices, Fetal Monitors, Neonatal Incubators, Prenatal Supplements |
| End User | Hospitals, Clinics, Home Care, Maternity Centers |
| Technology | Digital Imaging, Wireless Monitoring, Telemedicine, Wearable Devices |
| Application | Prenatal Care, Neonatal Intensive Care, Routine Check-ups, Emergency Services |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Spain Neonatal and Prenatal Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at