444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
The South Africa artificial organs and bionic implants market represents a transformative segment of the nation’s healthcare industry, characterized by rapid technological advancement and increasing medical demand. This specialized market encompasses sophisticated medical devices designed to replace or enhance human organ function, including artificial hearts, cochlear implants, prosthetic limbs, and various bionic enhancement systems. Market dynamics indicate substantial growth potential driven by an aging population, rising prevalence of chronic diseases, and advancing medical technology infrastructure.
Healthcare innovation in South Africa has positioned the country as a regional leader in medical device adoption and implementation. The market demonstrates remarkable resilience and growth trajectory, with annual growth rates consistently outpacing traditional medical device segments. Government initiatives supporting healthcare modernization and private sector investment in medical technology have created a conducive environment for market expansion.
Technological advancement continues to drive market evolution, with emerging solutions in neural interfaces, smart prosthetics, and bioengineered organ systems gaining significant traction. The integration of artificial intelligence and machine learning technologies into bionic implants has opened new possibilities for enhanced patient outcomes and improved quality of life.
The artificial organs and bionic implants market refers to the comprehensive ecosystem of medical devices, technologies, and services designed to replace, restore, or enhance human biological functions through artificial means. This market encompasses both temporary and permanent solutions, ranging from simple prosthetic devices to complex bioengineered organ systems that integrate seamlessly with human physiology.
Artificial organs specifically include devices that replicate the function of natural organs, such as artificial hearts, kidneys, lungs, and liver support systems. These sophisticated medical devices utilize advanced materials science, bioengineering principles, and cutting-edge manufacturing techniques to provide life-sustaining functionality for patients with organ failure or dysfunction.
Bionic implants represent a broader category of devices that enhance or restore human capabilities through technological integration. These include cochlear implants for hearing restoration, retinal implants for vision enhancement, neural stimulators for neurological conditions, and advanced prosthetic limbs with sensory feedback capabilities. The convergence of biotechnology, materials science, and digital innovation drives continuous advancement in this field.
Market expansion in South Africa’s artificial organs and bionic implants sector reflects the country’s commitment to healthcare innovation and technological advancement. The market demonstrates robust growth potential, supported by increasing healthcare expenditure, expanding medical infrastructure, and growing awareness of advanced treatment options among healthcare providers and patients.
Key growth drivers include the rising prevalence of cardiovascular diseases, diabetes-related complications, and age-related organ dysfunction. The market benefits from government support for healthcare modernization initiatives and increasing private healthcare investment. Regulatory frameworks have evolved to accommodate innovative medical technologies while ensuring patient safety and efficacy standards.
Technological integration represents a significant market differentiator, with South African healthcare facilities increasingly adopting advanced bionic implant systems. The market shows particular strength in cochlear implants, cardiac devices, and orthopedic implants, with emerging opportunities in neural interfaces and bioengineered organ systems. Regional positioning as a medical tourism destination further enhances market growth prospects.

Market segmentation reveals diverse opportunities across multiple medical specialties and patient demographics. The following key insights highlight critical market dynamics:
Demographic transformation serves as a primary market driver, with South Africa’s aging population creating increased demand for artificial organs and bionic implants. The prevalence of age-related organ dysfunction, cardiovascular disease, and diabetes continues to rise, necessitating advanced medical interventions and replacement therapies.
Healthcare infrastructure development significantly supports market growth through expanded medical facilities, specialized treatment centers, and enhanced surgical capabilities. Government investment in healthcare modernization and private sector expansion of medical services create favorable conditions for advanced medical device adoption.
Technological advancement drives market evolution through improved device functionality, enhanced biocompatibility, and reduced surgical complexity. Innovations in materials science, miniaturization, and wireless connectivity enable more sophisticated and user-friendly implant systems. Research collaboration between international manufacturers and local medical institutions accelerates technology transfer and adaptation.
Economic factors including expanded health insurance coverage and medical aid scheme benefits improve patient access to expensive artificial organ and implant procedures. Growing medical tourism industry positions South Africa as a regional hub for advanced medical procedures, attracting patients from neighboring countries seeking specialized treatments.
Cost considerations represent a significant market restraint, as artificial organs and bionic implants require substantial initial investment and ongoing maintenance expenses. Limited healthcare budgets and insurance coverage gaps restrict patient access to these advanced medical technologies, particularly in public healthcare settings.
Regulatory complexity creates challenges for market entry and product approval processes. Stringent safety requirements and clinical trial obligations extend development timelines and increase costs for manufacturers seeking to introduce innovative devices to the South African market. Regulatory harmonization with international standards remains an ongoing challenge.
Technical limitations in current artificial organ and implant technologies constrain market growth potential. Issues related to device longevity, biocompatibility, and integration with human physiology continue to present challenges for widespread adoption. Surgical complexity and specialized training requirements limit the number of healthcare providers capable of performing implantation procedures.
Infrastructure constraints in certain regions limit access to specialized medical facilities and post-operative care services. Uneven distribution of healthcare resources between urban and rural areas creates disparities in treatment availability and patient outcomes.
Emerging technologies present substantial opportunities for market expansion, particularly in areas of neural interfaces, bioengineered organs, and smart implant systems. Advances in 3D printing, tissue engineering, and nanotechnology open new possibilities for personalized medical devices and improved patient outcomes.
Regional expansion opportunities exist through South Africa’s position as a medical hub for sub-Saharan Africa. The country’s advanced healthcare infrastructure and medical expertise create potential for serving broader regional markets and establishing medical tourism programs focused on artificial organ and implant procedures.
Public-private partnerships offer opportunities for expanding access to artificial organs and bionic implants through innovative funding mechanisms and collaborative healthcare delivery models. Government initiatives supporting healthcare technology adoption and medical device manufacturing create favorable conditions for market growth.
Research and development opportunities abound through collaboration between international manufacturers, local universities, and medical research institutions. South Africa’s diverse patient population and clinical expertise provide valuable opportunities for device testing, refinement, and development of region-specific solutions.
Supply chain dynamics in the South African artificial organs and bionic implants market reflect a complex interplay of international manufacturers, local distributors, and healthcare providers. The market demonstrates increasing sophistication in logistics management, with specialized cold chain requirements and precision handling protocols ensuring device integrity and performance.
Competitive dynamics show intensifying competition among established medical device manufacturers and emerging technology companies. Market participants focus on differentiation through advanced features, improved patient outcomes, and comprehensive support services. Strategic partnerships between international companies and local healthcare providers facilitate market penetration and technology transfer.
Regulatory dynamics continue evolving to accommodate rapid technological advancement while maintaining rigorous safety standards. The South African Health Products Regulatory Authority (SAHPRA) works to streamline approval processes while ensuring comprehensive evaluation of new devices and technologies. International harmonization efforts facilitate faster market entry for globally approved devices.
Economic dynamics influence market accessibility and growth patterns, with currency fluctuations affecting import costs and pricing strategies. The market shows resilience through diversified supplier networks and local value-addition initiatives that reduce dependency on foreign exchange variations.
Comprehensive market analysis employs multiple research methodologies to ensure accurate and reliable market insights. Primary research involves extensive interviews with healthcare providers, medical device manufacturers, regulatory officials, and patient advocacy groups to gather firsthand market intelligence and trend identification.
Secondary research incorporates analysis of medical journals, regulatory filings, industry reports, and clinical trial databases to establish market baselines and identify emerging trends. Data triangulation methods ensure research accuracy through cross-verification of findings from multiple sources and methodologies.
Market modeling utilizes advanced statistical techniques and forecasting algorithms to project market trends and growth patterns. The methodology incorporates demographic analysis, epidemiological data, and healthcare utilization patterns to develop comprehensive market projections. Scenario analysis examines multiple market development pathways under different economic and regulatory conditions.
Stakeholder engagement throughout the research process ensures comprehensive understanding of market dynamics from multiple perspectives. Regular validation sessions with industry experts and healthcare professionals confirm research findings and enhance analytical accuracy.
Gauteng Province dominates the South African artificial organs and bionic implants market, accounting for approximately 45% of market activity due to its concentration of specialized medical facilities, research institutions, and healthcare infrastructure. The province benefits from proximity to major international airports, facilitating medical tourism and device importation logistics.
Western Cape represents the second-largest regional market with approximately 28% market share, driven by advanced cardiac surgery programs, research universities, and medical device manufacturing capabilities. The region’s strong medical tourism industry and international healthcare partnerships contribute to market growth and technology adoption.
KwaZulu-Natal shows emerging market potential with 15% market participation, supported by expanding private healthcare facilities and growing medical specialization programs. The province’s coastal location and developing medical infrastructure create opportunities for market expansion and patient access improvement.
Regional disparities in healthcare access and infrastructure create varying market dynamics across provinces. Urban centers demonstrate higher adoption rates and advanced technology utilization, while rural areas face challenges in accessing specialized medical services and implant procedures. Government initiatives aim to reduce these disparities through healthcare infrastructure development and telemedicine programs.
Market leadership in South Africa’s artificial organs and bionic implants sector involves both international manufacturers and specialized medical device companies. The competitive environment demonstrates increasing sophistication and technology advancement:
Product-based segmentation reveals diverse market opportunities across multiple medical device categories. The market demonstrates varying growth patterns and adoption rates across different product segments:
By Product Type:
By Application:
Cardiovascular devices maintain market leadership through established clinical protocols and proven patient outcomes. This category benefits from high disease prevalence and advanced cardiac surgery capabilities in South African medical centers. Innovation focus centers on miniaturization, wireless monitoring, and improved biocompatibility.
Neural implants represent the fastest-growing category with exceptional expansion potential driven by advancing neurotechnology and expanding clinical applications. Deep brain stimulation devices show particular promise for treating movement disorders and psychiatric conditions. Research collaboration between international manufacturers and local institutions accelerates development.
Prosthetic devices demonstrate strong market adoption through improved functionality and patient acceptance. Advanced bionic limbs with neural control interfaces show increasing demand among amputee populations. Customization capabilities and 3D printing technologies enhance device fit and performance.
Sensory restoration devices maintain steady growth through expanding patient eligibility and improved surgical techniques. Cochlear implants show particular strength in pediatric applications, while retinal implants emerge as promising solutions for vision restoration.
Healthcare providers benefit from enhanced treatment capabilities and improved patient outcomes through access to advanced artificial organs and bionic implants. These technologies enable medical professionals to offer life-saving and life-enhancing treatments previously unavailable, positioning facilities as centers of medical excellence and attracting specialized patient populations.
Patients and families experience transformative benefits through restored functionality, improved quality of life, and extended life expectancy. Advanced implant technologies provide opportunities for individuals with organ failure or physical disabilities to regain independence and participate fully in society. Long-term benefits include reduced healthcare costs and improved productivity.
Medical device manufacturers gain access to growing market opportunities and potential for technology advancement through collaboration with South African medical institutions. The market provides valuable clinical experience and patient data that inform product development and refinement. Regulatory approval in South Africa often facilitates broader African market entry.
Government and society benefit from reduced healthcare burden, improved population health outcomes, and enhanced medical tourism potential. Investment in artificial organs and bionic implants contributes to healthcare system modernization and positions South Africa as a regional medical leader.
Strengths:
Weaknesses:
Opportunities:
Threats:
Artificial intelligence integration represents a transformative trend in artificial organs and bionic implants, with smart devices capable of learning patient patterns and optimizing performance automatically. Machine learning algorithms enhance device functionality and predict maintenance needs, improving patient outcomes and reducing complications.
Miniaturization and wireless connectivity continue driving device innovation, enabling less invasive procedures and improved patient comfort. Wireless monitoring capabilities allow real-time device performance tracking and remote patient management, reducing healthcare costs and improving treatment adherence.
Personalized medicine approaches gain traction through 3D printing and custom device manufacturing. Patient-specific implants designed using individual anatomical data improve fit, function, and long-term outcomes. This trend particularly benefits prosthetic devices and orthopedic implants.
Bioengineering advancement focuses on developing more biocompatible materials and tissue-integrated devices. Regenerative medicine approaches combine artificial devices with biological components to create hybrid solutions that integrate more naturally with human physiology. Stem cell research contributes to organ regeneration possibilities.
Regulatory modernization initiatives by SAHPRA streamline approval processes for innovative medical devices while maintaining safety standards. Recent developments include expedited review pathways for breakthrough technologies and harmonization with international regulatory frameworks. Digital health regulations accommodate connected medical devices and remote monitoring systems.
Research partnerships between international manufacturers and South African institutions accelerate technology development and clinical validation. Notable collaborations include neural interface research programs and artificial organ development initiatives. Clinical trial expansion provides South African patients access to cutting-edge treatments while contributing to global research efforts.
Infrastructure investment in specialized medical facilities enhances the country’s capacity for complex implant procedures. New cardiac surgery centers and neural intervention facilities expand treatment availability and attract medical tourism. Training programs for healthcare professionals ensure adequate expertise for advanced procedures.
Manufacturing initiatives explore local production capabilities for certain medical device components, reducing costs and improving supply chain resilience. Technology transfer programs facilitate knowledge sharing and skill development in medical device manufacturing and maintenance.
Market participants should focus on developing comprehensive patient support programs that address the entire treatment journey from initial consultation through long-term follow-up care. MarkWide Research analysis indicates that successful market penetration requires strong clinical support and patient education initiatives.
Investment priorities should emphasize technology advancement in areas of artificial intelligence, wireless connectivity, and biocompatible materials. Companies investing in next-generation technologies position themselves for long-term market leadership and competitive advantage. Research and development collaboration with local institutions provides valuable market insights and clinical validation opportunities.
Market access strategies should incorporate innovative funding mechanisms and partnership models to address cost barriers and expand patient access. Public-private partnerships and medical aid scheme negotiations can significantly improve market penetration and patient outcomes.
Regional expansion opportunities should be pursued through South Africa’s position as a medical hub for sub-Saharan Africa. Establishing regional distribution networks and training programs can capture broader market opportunities while contributing to healthcare development across the continent.
Market trajectory for South Africa’s artificial organs and bionic implants sector indicates sustained growth driven by technological advancement, demographic trends, and healthcare infrastructure development. MWR projections suggest continued expansion across all major product categories with particular strength in neural implants and smart prosthetic devices.
Technology evolution will focus on enhanced biointegration, improved longevity, and reduced surgical complexity. Emerging technologies including brain-computer interfaces, bioengineered organs, and nanotechnology-enhanced devices will create new treatment possibilities and market opportunities. Artificial intelligence integration will become standard across device categories.
Market accessibility improvements through innovative funding models, insurance coverage expansion, and cost reduction initiatives will broaden patient access to advanced treatments. Government support for healthcare modernization and medical device manufacturing will contribute to market growth and technology adoption.
Regional leadership position will strengthen through continued investment in medical infrastructure, research capabilities, and healthcare professional training. South Africa’s role as a medical tourism destination and regional healthcare hub will drive sustained market expansion and international recognition.
South Africa’s artificial organs and bionic implants market represents a dynamic and rapidly evolving sector with substantial growth potential and transformative impact on healthcare delivery. The market demonstrates remarkable resilience and innovation capacity, supported by advanced medical infrastructure, skilled healthcare professionals, and progressive regulatory frameworks.
Key success factors include continued investment in technology advancement, expanded patient access through innovative funding mechanisms, and strengthened partnerships between international manufacturers and local healthcare providers. The market’s position as a regional leader creates opportunities for broader African market development and medical tourism growth.
Future prospects remain highly positive, with emerging technologies, demographic trends, and healthcare modernization initiatives driving sustained market expansion. The convergence of artificial intelligence, bioengineering, and personalized medicine approaches will create new possibilities for patient treatment and improved outcomes, positioning South Africa as a global leader in artificial organs and bionic implants innovation and implementation.
What is Artificial Organs & Bionic Implants?
Artificial organs and bionic implants are medical devices designed to replace or enhance biological functions in the human body. They include products like artificial hearts, bionic limbs, and retinal implants, which aim to improve the quality of life for patients with severe health conditions.
What are the key players in the South Africa Artificial Organs & Bionic Implants Market?
Key players in the South Africa Artificial Organs & Bionic Implants Market include companies like Medtronic, Boston Scientific, and Stryker, which are known for their innovative medical technologies and devices. These companies focus on developing advanced solutions for organ replacement and bionic enhancements, among others.
What are the growth factors driving the South Africa Artificial Organs & Bionic Implants Market?
The South Africa Artificial Organs & Bionic Implants Market is driven by factors such as the increasing prevalence of chronic diseases, advancements in medical technology, and a growing aging population. These elements contribute to a higher demand for effective medical solutions and improved patient outcomes.
What challenges does the South Africa Artificial Organs & Bionic Implants Market face?
Challenges in the South Africa Artificial Organs & Bionic Implants Market include high costs of advanced technologies, regulatory hurdles, and the need for skilled professionals to implement these solutions. Additionally, patient acceptance and the risk of complications can hinder market growth.
What opportunities exist in the South Africa Artificial Organs & Bionic Implants Market?
Opportunities in the South Africa Artificial Organs & Bionic Implants Market include the potential for innovation in biocompatible materials and the development of personalized medical devices. Furthermore, increasing investment in healthcare infrastructure can enhance access to these technologies.
What trends are shaping the South Africa Artificial Organs & Bionic Implants Market?
Trends in the South Africa Artificial Organs & Bionic Implants Market include the rise of minimally invasive surgical techniques and the integration of smart technology in bionic implants. These trends aim to improve patient recovery times and enhance the functionality of artificial organs.
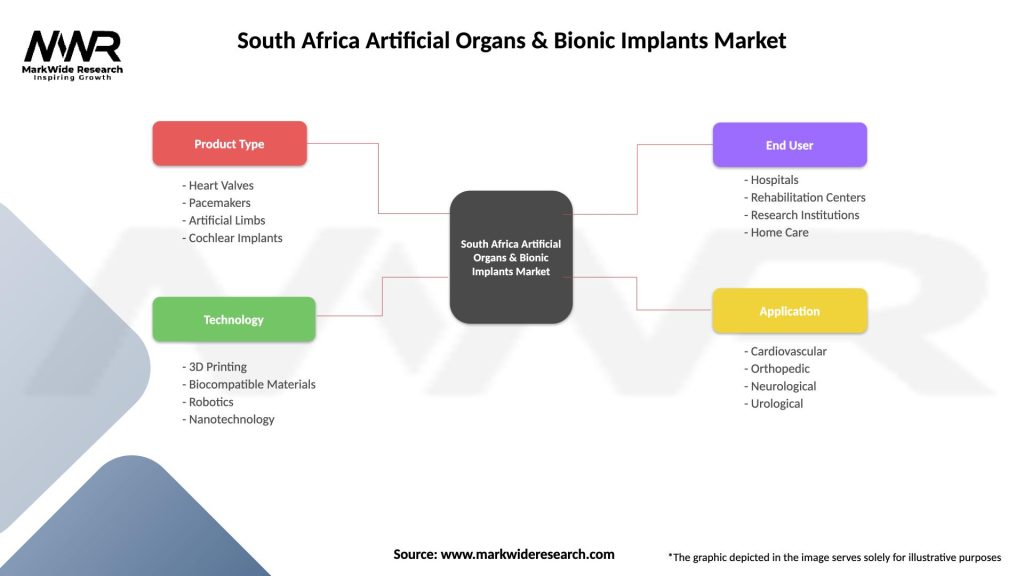
South Africa Artificial Organs & Bionic Implants Market
| Segmentation Details | Description |
|---|---|
| Product Type | Heart Valves, Pacemakers, Artificial Limbs, Cochlear Implants |
| Technology | 3D Printing, Biocompatible Materials, Robotics, Nanotechnology |
| End User | Hospitals, Rehabilitation Centers, Research Institutions, Home Care |
| Application | Cardiovascular, Orthopedic, Neurological, Urological |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the South Africa Artificial Organs & Bionic Implants Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at