444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The Respiratory Syncytial Virus (RSV) Therapeutics Market is experiencing significant growth and is expected to continue expanding in the coming years. RSV is a common respiratory virus that affects individuals of all ages, but it is particularly severe in infants, older adults, and individuals with compromised immune systems. RSV infections can lead to serious respiratory illnesses such as bronchiolitis and pneumonia, making the development of effective therapeutics crucial.
Respiratory Syncytial Virus (RSV) is a viral infection that affects the respiratory system, primarily targeting the lungs and airways. It is a leading cause of respiratory illness in children and infants. RSV can cause symptoms ranging from mild cold-like symptoms to severe respiratory distress. Due to the significant impact of RSV infections, the development of therapeutics to prevent and treat the virus is of utmost importance.
Executive Summary
The global Respiratory Syncytial Virus (RSV) Therapeutics Market is witnessing robust growth due to the increasing prevalence of RSV infections and the rising demand for effective treatment options. The market is characterized by ongoing research and development activities aimed at developing novel therapeutics to combat RSV. Key players in the market are focusing on strategic collaborations, partnerships, and acquisitions to expand their product portfolios and gain a competitive edge.
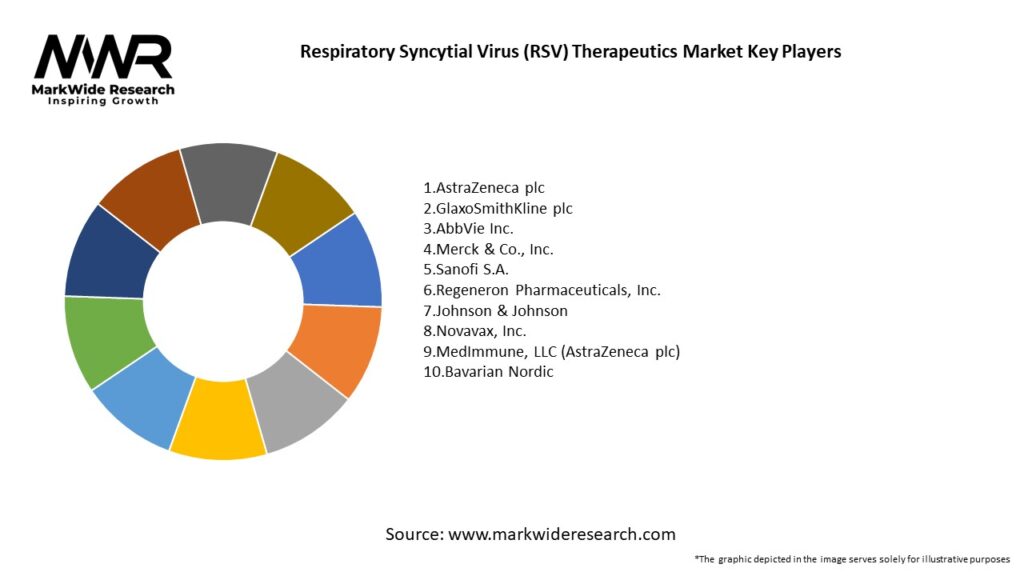
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
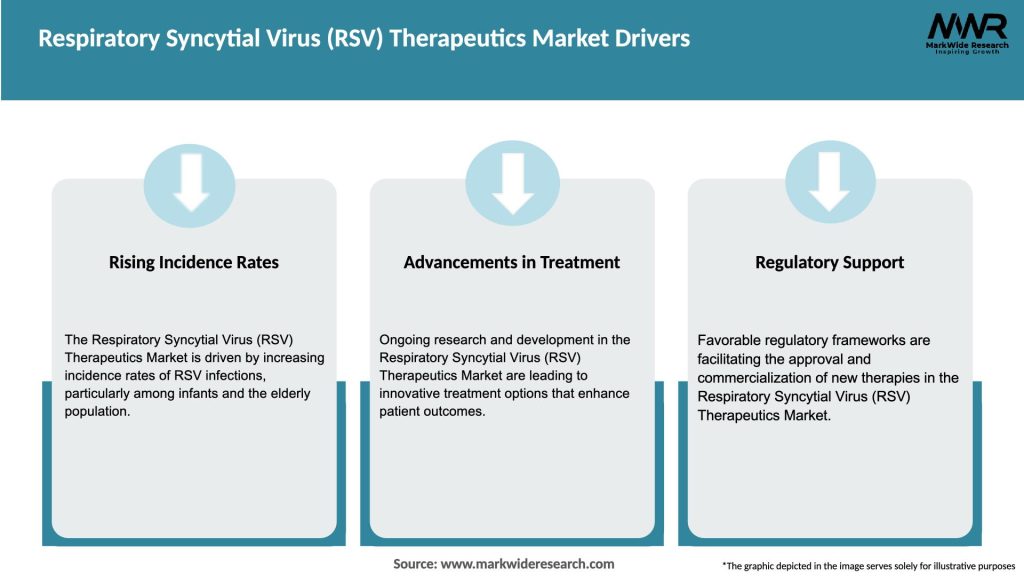
Market Drivers
The following factors are driving the growth of the Respiratory Syncytial Virus (RSV) Therapeutics Market:
Market Restraints
Despite the positive market outlook, certain factors may hinder the growth of the Respiratory Syncytial Virus (RSV) Therapeutics Market:
Market Opportunities
The Respiratory Syncytial Virus (RSV) Therapeutics Market presents several opportunities for growth:
Market Dynamics
The Respiratory Syncytial Virus (RSV) Therapeutics Market is driven by a combination of factors, including the increasing prevalence of RSV infections, advancements in technology, and favorable government initiatives. However, challenges such as high treatment costs and stringent regulatory requirements may impede market growth. Nevertheless, ongoing research and development activities
Regional Analysis
The Respiratory Syncytial Virus (RSV) Therapeutics Market can be analyzed based on regional segmentation:
Competitive Landscape
Leading Companies in the Respiratory Syncytial Virus (RSV) Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
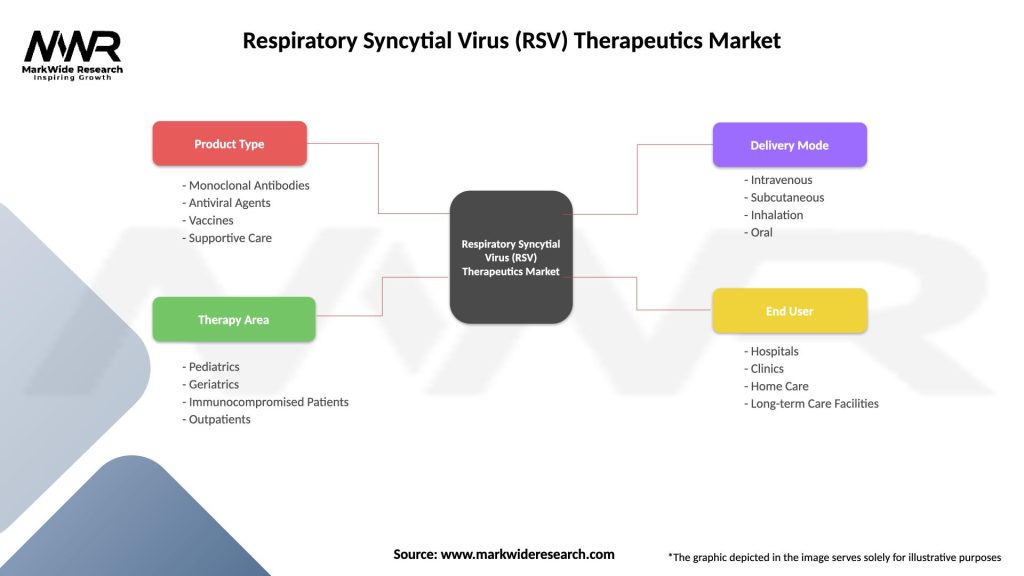
Segmentation
The Respiratory Syncytial Virus (RSV) Therapeutics Market can be segmented based on the following criteria:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the Respiratory Syncytial Virus (RSV) Therapeutics Market. Due to the focus on managing and mitigating the spread of Covid-19, there has been a potential decline in RSV infections in certain regions. However, it is important to note that the impact of Covid-19 on RSV epidemiology and the therapeutic market is still being studied.
The measures taken to prevent the spread of Covid-19, such as social distancing, wearing masks, and improved hygiene practices, have also inadvertently contributed to a decrease in RSV transmission. As a result, the demand for RSV therapeutics may have been temporarily affected.
However, as the world gradually recovers from the pandemic, it is expected that RSV infections will rebound, creating renewed demand for effective therapeutics. The lessons learned from managing Covid-19 may also contribute to improved strategies for RSV prevention, diagnosis.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the Respiratory Syncytial Virus (RSV) Therapeutics Market looks promising, driven by ongoing research and development activities, increasing investments, and advancements in technology. The market is expected to witness the introduction of innovative therapeutics, including antiviral agents, monoclonal antibodies, and vaccines. The focus on personalized medicine and the integration of artificial intelligence and machine learning will further enhance RSV treatment approaches.
Additionally, as the impact of Covid-19 subsides, the demand for RSV therapeutics is projected to rebound. The growing awareness about the severity of RSV infections and the need for effective treatment options will drive market growth.
Conclusion
The Respiratory Syncytial Virus (RSV) Therapeutics Market is poised for significant growth in the coming years. With a focus on developing innovative therapeutics, expanding into untapped markets, and addressing unmet needs, industry players can capitalize on the increasing demand for effective RSV treatments. Collaborative partnerships, advancements in technology, and adherence to regulatory compliance will be key factors in driving market success. The future outlook for the RSV therapeutics market is promising, with opportunities for improved patient outcomes and advancements in the field of respiratory virus management.
The Respiratory Syncytial Virus (RSV) Therapeutics Market is expected to witness significant growth in the coming years. The increasing prevalence of RSV infections, coupled with rising awareness about the severity of the disease, is driving the demand for effective therapeutics. Key market players are actively engaged in research and development activities to develop innovative antiviral agents, monoclonal antibodies, and vaccines to combat RSV.
What is Respiratory Syncytial Virus (RSV) therapeutics?
Respiratory Syncytial Virus (RSV) therapeutics refer to the medical treatments and interventions designed to prevent or manage infections caused by the RSV, which primarily affects infants and young children, leading to respiratory illnesses.
What are the key companies in the Respiratory Syncytial Virus (RSV) therapeutics market?
Key companies in the Respiratory Syncytial Virus (RSV) therapeutics market include AbbVie, AstraZeneca, and Sanofi, among others.
What are the main drivers of growth in the Respiratory Syncytial Virus (RSV) therapeutics market?
The main drivers of growth in the Respiratory Syncytial Virus (RSV) therapeutics market include the increasing incidence of RSV infections, rising awareness about the disease, and advancements in treatment options.
What challenges does the Respiratory Syncytial Virus (RSV) therapeutics market face?
Challenges in the Respiratory Syncytial Virus (RSV) therapeutics market include the high cost of drug development, regulatory hurdles, and competition from alternative treatments.
What opportunities exist in the Respiratory Syncytial Virus (RSV) therapeutics market?
Opportunities in the Respiratory Syncytial Virus (RSV) therapeutics market include the development of novel therapies, potential for combination treatments, and expanding research into preventive vaccines.
What trends are shaping the Respiratory Syncytial Virus (RSV) therapeutics market?
Trends shaping the Respiratory Syncytial Virus (RSV) therapeutics market include increased investment in biotechnology, a focus on personalized medicine, and the integration of digital health technologies in treatment protocols.
Respiratory Syncytial Virus (RSV) Therapeutics Market
| Segmentation Details | Description |
|---|---|
| Product Type | Monoclonal Antibodies, Antiviral Agents, Vaccines, Supportive Care |
| Therapy Area | Pediatrics, Geriatrics, Immunocompromised Patients, Outpatients |
| Delivery Mode | Intravenous, Subcutaneous, Inhalation, Oral |
| End User | Hospitals, Clinics, Home Care, Long-term Care Facilities |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Respiratory Syncytial Virus (RSV) Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at