444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The rescue airway devices market is witnessing significant growth globally, driven by the increasing prevalence of respiratory emergencies, advancements in airway management techniques, and growing demand for portable and user-friendly emergency medical devices. Rescue airway devices are specialized tools used by healthcare professionals to establish and maintain a patient’s airway during critical situations such as cardiac arrest, trauma, respiratory failure, and anesthesia induction.
Meaning
Rescue airway devices, also known as supraglottic airway devices or advanced airway management devices, are medical devices designed to facilitate ventilation and oxygenation in patients who require emergency airway management. These devices are inserted into the patient’s airway to secure the passage of air and prevent airway obstruction, allowing for effective respiratory support during critical situations where traditional endotracheal intubation may be challenging or contraindicated.
Executive Summary
The global rescue airway devices market is experiencing rapid growth, driven by the increasing demand for pre-hospital emergency care, advancements in airway management technology, and rising awareness about the importance of timely intervention in respiratory emergencies. Key market players are focusing on product innovation, quality improvement, and strategic partnerships to expand their product portfolios and gain a competitive edge in the market.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several factors are driving the growth of the rescue airway devices market, including:
Market Restraints
Despite the promising growth prospects, the rescue airway devices market faces certain challenges, such as:
Market Opportunities
The rescue airway devices market presents several opportunities for growth and innovation, including:
Market Dynamics
The rescue airway devices market is characterized by dynamic trends, including:
Regional Analysis
North America dominates the global rescue airway devices market, driven by well-established emergency medical services (EMS) infrastructure, trauma care systems, and pre-hospital emergency care protocols. Europe follows closely, supported by robust regulatory framework, clinical practice guidelines, and quality assurance standards for emergency airway management. Asia-Pacific is poised for significant growth, fueled by increasing investments in healthcare infrastructure, rising demand for critical care services, and expanding access to pre-hospital emergency care in urban and rural areas.
Competitive Landscape
Leading Companies in Rescue Airway Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
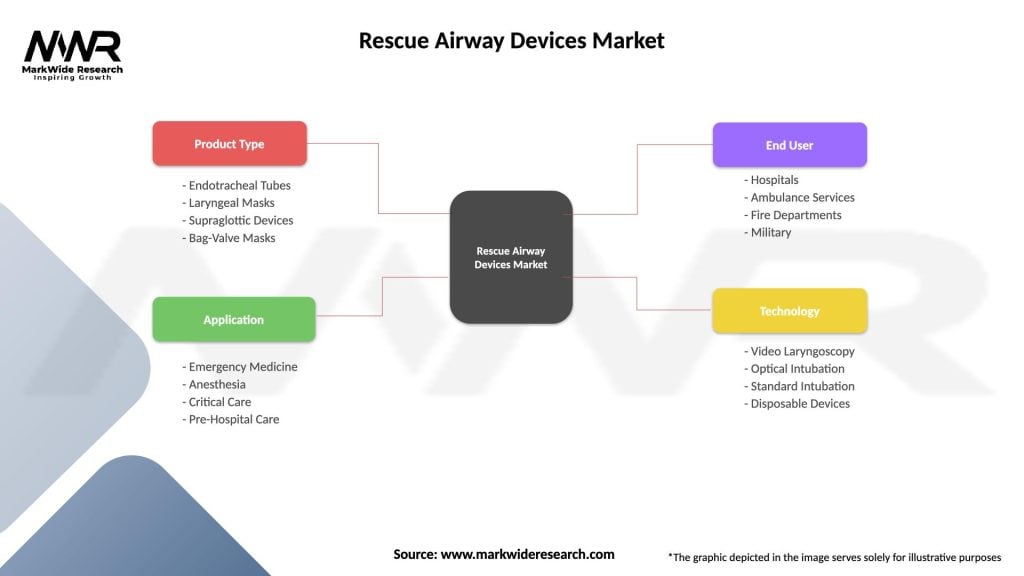
Segmentation
The rescue airway devices market can be segmented based on:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The rescue airway devices market offers several benefits for industry participants and stakeholders, including:
SWOT Analysis
A SWOT analysis of the rescue airway devices market reveals the following:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the rescue airway devices market, with increased demand for emergency medical services, critical care transport, and respiratory support in patients with severe respiratory failure due to Covid-19 infection. Rescue airway devices, including supraglottic airway devices, video laryngoscopes, and disposable laryngeal mask airways, have played a crucial role in airway management and ventilatory support in Covid-19 patients, particularly during airway emergencies, intubation procedures, and prone positioning maneuvers. However, supply chain disruptions, shortages of personal protective equipment (PPE), and challenges in healthcare resource allocation have posed logistical challenges for emergency medical services (EMS) agencies, hospitals, and critical care units responding to the pandemic.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the rescue airway devices market is optimistic, driven by increasing demand for emergency medical services, advancements in airway management technology, and rising awareness about the importance of timely intervention in respiratory emergencies. As the global population grows, and the burden of trauma, cardiac arrest, and respiratory diseases increases, there will be growing demand for portable, user-friendly, and technologically advanced rescue airway devices in pre-hospital and critical care settings. Telemedicine, mobile health, and remote monitoring technologies are expected to play an increasingly important role in airway management, patient monitoring, and quality assurance in emergency medical services (EMS) and critical care transport. However, addressing challenges such as variability in clinical performance, user training gaps, and regulatory hurdles will be essential to realizing the full potential of the rescue airway devices market in improving patient outcomes and saving lives in emergencies.
Conclusion
In conclusion, the rescue airway devices market is experiencing rapid growth and innovation, driven by increasing demand for emergency medical services, advancements in airway management technology, and rising awareness about the importance of timely intervention in respiratory emergencies. By prioritizing airway assessment, intervention, and management protocols, healthcare providers can improve patient outcomes and save lives in critical situations. Collaboration among industry stakeholders, regulatory authorities, and research institutions will be crucial in addressing challenges and realizing the full potential of rescue airway devices in supporting emergency medical services (EMS), critical care transport, and disaster response on a global scale.
What is Rescue Airway Devices?
Rescue airway devices are medical tools designed to secure and maintain an open airway in patients who are unable to breathe independently. These devices are critical in emergency situations, particularly in pre-hospital settings and during surgical procedures.
What are the key players in the Rescue Airway Devices Market?
Key players in the Rescue Airway Devices Market include Medtronic, Smiths Medical, and Teleflex, among others. These companies are known for their innovative products and contributions to airway management solutions.
What are the main drivers of growth in the Rescue Airway Devices Market?
The growth of the Rescue Airway Devices Market is driven by the increasing incidence of respiratory emergencies, advancements in medical technology, and the rising awareness of effective airway management techniques. Additionally, the growing number of emergency medical services is contributing to market expansion.
What challenges does the Rescue Airway Devices Market face?
The Rescue Airway Devices Market faces challenges such as the high cost of advanced airway management devices and the need for specialized training for healthcare professionals. Furthermore, regulatory hurdles can also impact the speed of product development and market entry.
What opportunities exist in the Rescue Airway Devices Market?
Opportunities in the Rescue Airway Devices Market include the development of innovative, user-friendly devices and the expansion of telemedicine services that facilitate remote airway management. Additionally, increasing investments in healthcare infrastructure present further growth potential.
What trends are shaping the Rescue Airway Devices Market?
Trends in the Rescue Airway Devices Market include the integration of smart technology in airway devices, such as sensors and connectivity features, to enhance patient monitoring. There is also a growing emphasis on training and simulation programs to improve the skills of healthcare providers in airway management.
Rescue Airway Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Endotracheal Tubes, Laryngeal Masks, Supraglottic Devices, Bag-Valve Masks |
| Application | Emergency Medicine, Anesthesia, Critical Care, Pre-Hospital Care |
| End User | Hospitals, Ambulance Services, Fire Departments, Military |
| Technology | Video Laryngoscopy, Optical Intubation, Standard Intubation, Disposable Devices |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Rescue Airway Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at