444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The reprocessed medical devices market refers to the reprocessing or refurbishment of single-use medical devices (SUDs) for their safe and effective reuse. Reprocessing involves cleaning, disinfection, testing, and refurbishing these devices to meet the required standards. The market for reprocessed medical devices has gained significant traction in recent years, driven by cost containment measures, environmental sustainability concerns, and the need to reduce medical waste. Reprocessed medical devices offer a cost-effective alternative to new devices without compromising patient safety and quality of care.
Meaning
Reprocessed medical devices are previously used single-use medical devices that undergo a rigorous reprocessing process to ensure they meet the necessary safety and efficacy standards for reuse. These devices are thoroughly cleaned, disinfected, and tested to ensure they are free from contaminants and meet the required performance specifications. Reprocessing allows healthcare facilities to reduce costs associated with the purchase of new devices while maintaining the same level of quality and safety.
Executive Summary
The reprocessed medical devices market has experienced significant growth in recent years. The increasing demand for cost-effective healthcare solutions, growing concerns regarding environmental sustainability, and the need to reduce medical waste have fueled the adoption of reprocessed medical devices. The market offers healthcare providers an opportunity to reduce expenses without compromising patient safety. However, stringent regulations and the perception of inferior quality associated with reprocessed devices pose challenges to market growth. Despite these challenges, the reprocessed medical devices market is expected to continue its expansion due to the cost-saving potential and environmental benefits it offers.
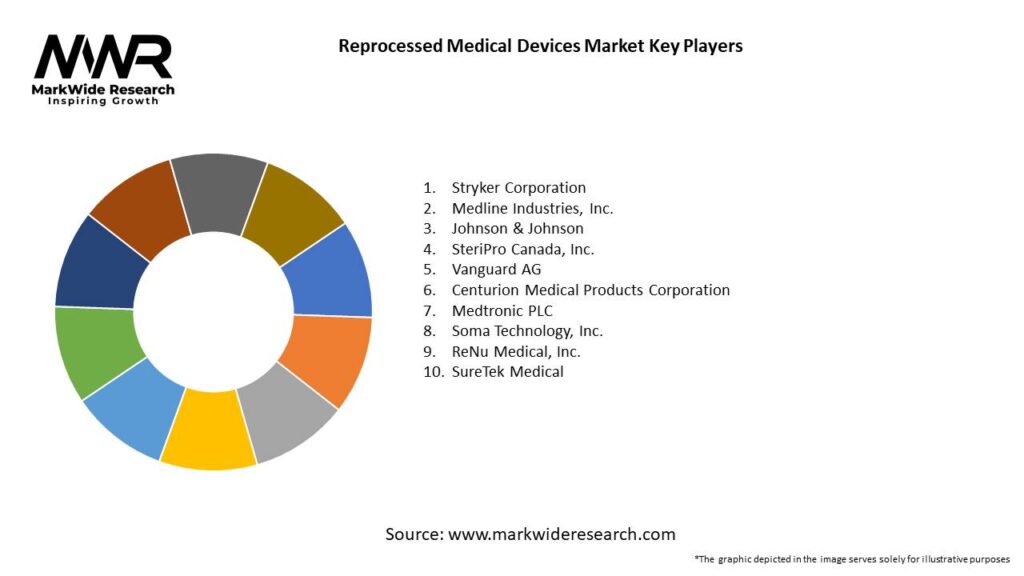
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
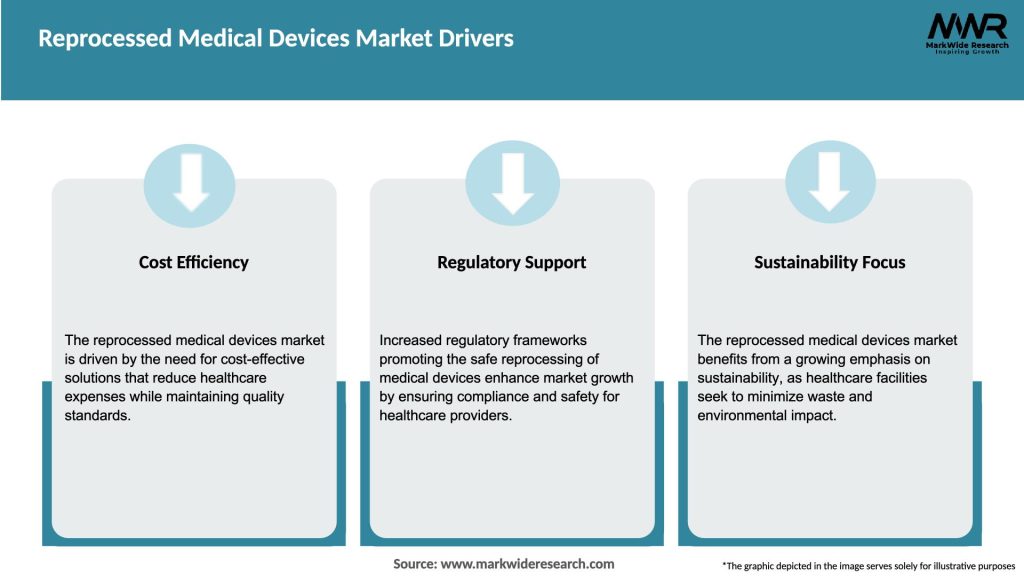
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The reprocessed medical devices market is dynamic and influenced by various factors. The increasing focus on cost containment, environmental sustainability, and patient safety drives market growth. However, challenges such as the perception of inferior quality and stringent regulatory requirements need to be addressed to unlock the full potential of the market. Opportunities lie in expanding market penetration in developing countries, technological advancements, and strategic collaborations. Continuous market monitoring and adaptation to changing dynamics will be essential for sustained growth in the reprocessed medical devices market.
Regional Analysis
The reprocessed medical devices market exhibits regional variations due to differences in healthcare infrastructure, regulatory frameworks, and market dynamics. North America, with its well-established healthcare system and stringent regulatory environment, has been a significant market for reprocessed medical devices. Europe has also witnessed substantial growth, driven by cost containment measures and environmental sustainability initiatives. Developing regions such as Asia-Pacific and Latin America offer significant growth potential due to the increasing adoption of reprocessed devices to optimize healthcare expenditure. Regional variations in market size, regulatory requirements, and consumer preferences should be considered for successful market entry and expansion.
Competitive Landscape
Leading Companies in Reprocessed Medical Devices Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
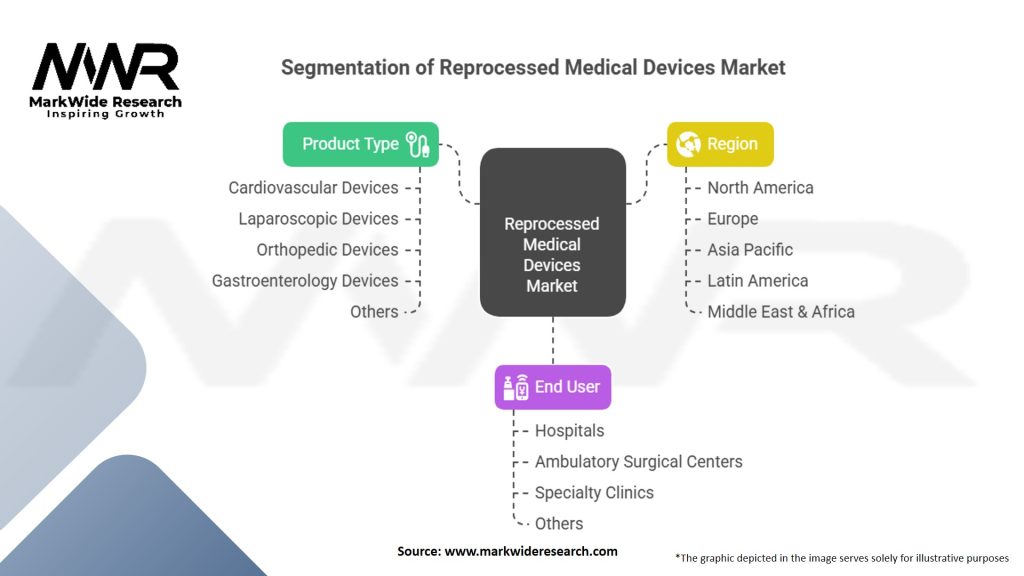
Segmentation
The reprocessed medical devices market can be segmented based on various factors, including:
Category-wise Insights
Reprocessed medical devices can be categorized into various categories based on their application and complexity. Some of the key categories include:
Categorizing reprocessed medical devices allows for a focused understanding of the unique considerations and market dynamics associated with each category, aiding market players in developing tailored strategies.
Key Benefits for Industry Participants and Stakeholders
The reprocessed medical devices market offers several key benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT analysis provides a comprehensive assessment of the strengths, weaknesses, opportunities, and threats in the reprocessed medical devices market:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a mixed impact on the reprocessed medical devices market. On one hand, the increased focus on infection control and prevention has raised concerns about the reusability of medical devices, leading to a temporary decline in the adoption of reprocessed devices. Additionally, disruptions in the supply chain and healthcare operations during the pandemic have impacted the availability and utilization of reprocessed devices.
On the other hand, the economic strain caused by the pandemic has highlighted the need for cost containment measures in the healthcare industry. Reprocessed medical devices have the potential to offer significant cost savings, making them an attractive option for healthcare providers facing financial constraints. Moreover, the emphasis on environmental sustainability and reduced medical waste aligns with the principles promoted during the pandemic, driving the adoption of reprocessed devices.
Key Industry Developments
Analyst Suggestions
Future Outlook
The reprocessed medical devices market is expected to witness sustained growth in the coming years. The increasing focus on cost containment, environmental sustainability, and patient safety will continue to drive the adoption of reprocessed devices. However, addressing concerns related to quality perception, overcoming regulatory challenges, and expanding the range of devices suitable for reprocessing will be crucial for unlocking the full potential of the market. Continued technological advancements, strategic partnerships, and market education initiatives will contribute to the future growth and acceptance of reprocessed medical devices.
Conclusion
The reprocessed medical devices market offers cost-effective solutions to healthcare providers while addressing concerns related to environmental sustainability. Despite challenges such as quality perception and regulatory requirements, the market continues to expand, driven by cost containment measures, the need for sustainable healthcare practices, and the potential for improved access to quality care. The COVID-19 pandemic has both impacted and highlighted the significance of reprocessed devices in the healthcare industry. As the market evolves, collaborations, technological advancements, and education initiatives will play pivotal roles in shaping the future of the reprocessed medical devices market.
What is Reprocessed Medical Devices?
Reprocessed medical devices are previously used medical instruments that have been cleaned, refurbished, and sterilized for safe reuse. This practice helps reduce waste and lower healthcare costs while maintaining safety standards.
What are the key players in the Reprocessed Medical Devices Market?
Key players in the Reprocessed Medical Devices Market include Stryker Corporation, Medline Industries, and ReNu Medical, among others. These companies focus on providing high-quality reprocessed devices across various medical fields.
What are the main drivers of the Reprocessed Medical Devices Market?
The main drivers of the Reprocessed Medical Devices Market include the rising demand for cost-effective healthcare solutions, increasing focus on sustainability in healthcare, and advancements in sterilization technologies. These factors contribute to the growing acceptance of reprocessed devices.
What challenges does the Reprocessed Medical Devices Market face?
The Reprocessed Medical Devices Market faces challenges such as regulatory hurdles, concerns regarding the safety and efficacy of reused devices, and competition from new, single-use products. These factors can hinder market growth and acceptance.
What opportunities exist in the Reprocessed Medical Devices Market?
Opportunities in the Reprocessed Medical Devices Market include expanding into emerging markets, increasing partnerships with healthcare facilities, and developing innovative reprocessing technologies. These avenues can enhance market penetration and growth.
What trends are shaping the Reprocessed Medical Devices Market?
Trends shaping the Reprocessed Medical Devices Market include a growing emphasis on environmental sustainability, increased regulatory scrutiny, and the adoption of advanced technologies for reprocessing. These trends are influencing how healthcare providers approach device reuse.
Reprocessed Medical Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Cardiovascular Devices, Laparoscopic Devices, Orthopedic Devices, Gastroenterology Devices, Others |
| End User | Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Reprocessed Medical Devices Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at