444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Remdesivir market has witnessed significant growth due to the outbreak of the COVID-19 pandemic. Remdesivir, an antiviral medication, gained prominence as a potential treatment for COVID-19 patients. It has been approved for emergency use by several regulatory authorities across the globe. The market for Remdesivir has experienced a surge in demand, with pharmaceutical companies ramping up production to meet the needs of healthcare systems worldwide.
Meaning
Remdesivir is a broad-spectrum antiviral drug developed by Gilead Sciences. Originally designed to treat Ebola virus disease, it has shown promise as a potential treatment for COVID-19. Remdesivir works by inhibiting viral RNA replication, which can help reduce the severity and duration of symptoms in COVID-19 patients. It is administered intravenously and is typically used for hospitalized patients with severe cases of the disease.
Executive Summary
The Remdesivir market has witnessed unprecedented growth in the wake of the COVID-19 pandemic. The rapid spread of the virus and the urgent need for effective treatments have driven the demand for Remdesivir. Pharmaceutical companies have focused their efforts on scaling up production to meet the global requirements. The market is expected to continue its growth trajectory as the world grapples with the ongoing challenges posed by the pandemic.
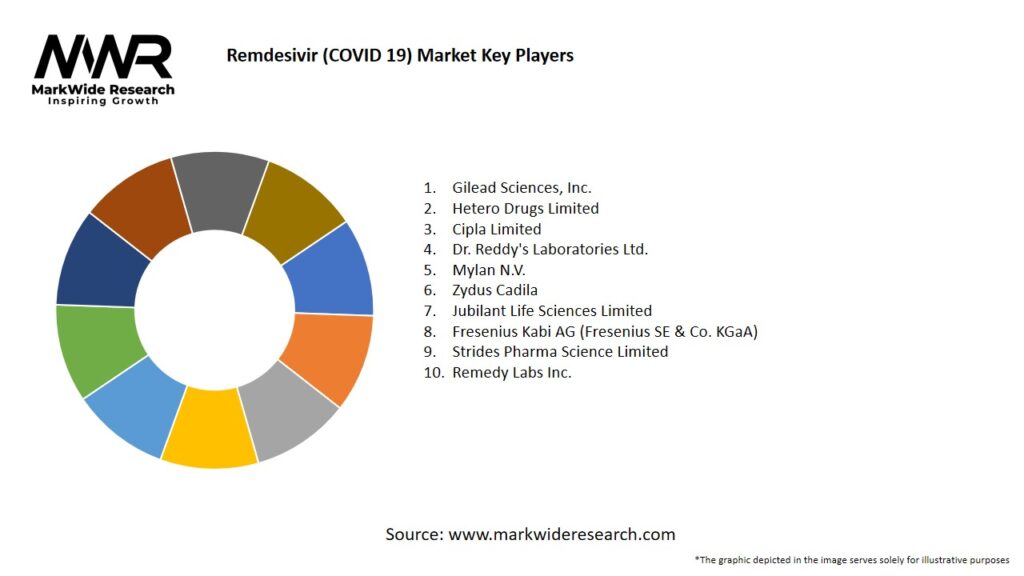
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Remdesivir market is driven by the urgent need for effective treatments for COVID-19. The market dynamics are influenced by factors such as regulatory approvals, research and development initiatives, supply chain management, and pricing strategies. The evolving nature of the pandemic and the emergence of new variants of the virus also impact the market dynamics.
Regional Analysis
The market for Remdesivir is global in scope, with demand spanning across various regions. North America, Europe, Asia Pacific, and the Rest of the World are key regions contributing to the market growth. Factors such as healthcare infrastructure, regulatory environment, and the prevalence of COVID-19 cases influence the regional demand for Remdesivir.
Competitive Landscape
Leading Companies in the Remdesivir (COVID 19) Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Remdesivir market can be segmented based on:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has significantly impacted the Remdesivir market. The demand for the drug skyrocketed due to its potential as a treatment option for COVID-19 patients. The market experienced supply chain disruptions initially, but pharmaceutical companies swiftly adapted to meet the surge in demand. Regulatory approvals and emergency use authorizations played a crucial role in facilitating the widespread use of Remdesivir for COVID-19 treatment.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Remdesivir market is expected to witness continued growth in the coming years, driven by the persistent global impact of COVID-19. The market dynamics will be influenced by factors such as regulatory developments, clinical trial outcomes, supply chain management, and pricing strategies.
Conclusion
In conclusion, the Remdesivir market has experienced substantial growth and demand due to its potential as a treatment for COVID-19. The urgency to find effective solutions for managing the pandemic has led to regulatory approvals and emergency use authorizations, allowing widespread deployment of Remdesivir. Pharmaceutical companies have scaled up production capacities to meet the global requirements. The market’s future outlook remains positive, with ongoing research, expanding manufacturing capacities, and the exploration of combination therapies. However, challenges such as supply chain disruptions and high costs need to be addressed to ensure wider accessibility. The Remdesivir market continues to evolve, driven by the ever-changing dynamics of the COVID-19 pandemic and the need for effective treatment options.
What is Remdesivir?
Remdesivir is an antiviral medication that has been used in the treatment of COVID-19. It works by inhibiting the replication of the virus, making it a critical option in managing severe cases of the disease.
What are the key players in the Remdesivir (COVID 19) market?
Key players in the Remdesivir (COVID 19) market include Gilead Sciences, which developed the drug, as well as other pharmaceutical companies involved in its distribution and production, such as Mylan and Cipla, among others.
What are the growth factors driving the Remdesivir (COVID 19) market?
The growth of the Remdesivir (COVID 19) market is driven by the ongoing need for effective COVID-19 treatments, increasing hospitalization rates, and the emergence of new variants that may require antiviral therapies.
What challenges does the Remdesivir (COVID 19) market face?
Challenges in the Remdesivir (COVID 19) market include supply chain disruptions, competition from other antiviral treatments, and concerns regarding the drug’s efficacy against certain variants of the virus.
What opportunities exist in the Remdesivir (COVID 19) market?
Opportunities in the Remdesivir (COVID 19) market include potential expansions into new therapeutic areas, ongoing research for combination therapies, and the development of generics as patents expire.
What trends are shaping the Remdesivir (COVID 19) market?
Trends in the Remdesivir (COVID 19) market include increased collaboration between pharmaceutical companies and governments, advancements in drug delivery systems, and a focus on real-world evidence to support treatment protocols.
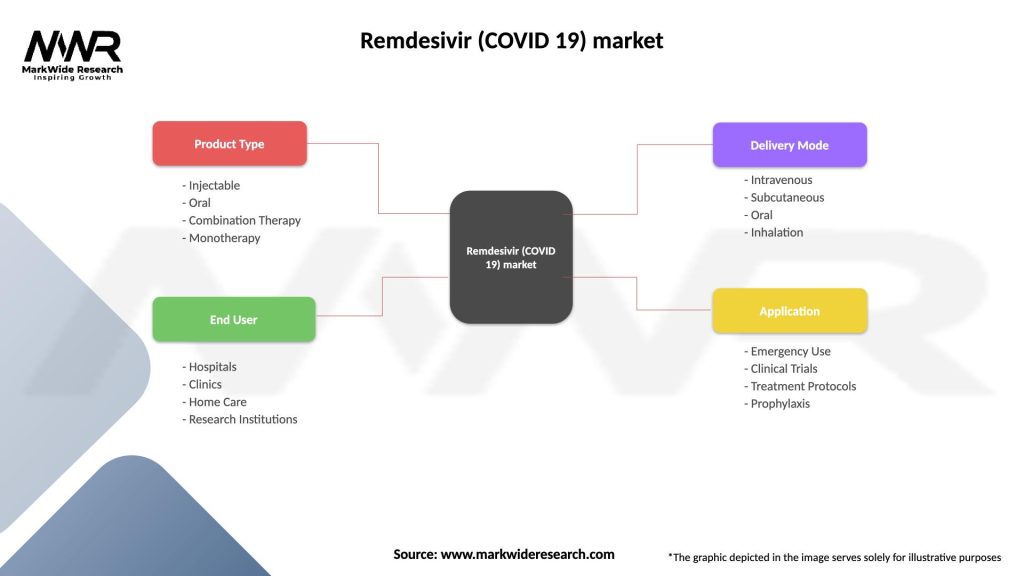
Remdesivir (COVID 19) market
| Segmentation Details | Description |
|---|---|
| Product Type | Injectable, Oral, Combination Therapy, Monotherapy |
| End User | Hospitals, Clinics, Home Care, Research Institutions |
| Delivery Mode | Intravenous, Subcutaneous, Oral, Inhalation |
| Application | Emergency Use, Clinical Trials, Treatment Protocols, Prophylaxis |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Remdesivir (COVID 19) Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at