444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The Recombinant Vector Vaccine Market is experiencing significant growth and is poised to expand even further in the coming years. This market analysis will provide insights into the current scenario, key trends, drivers, restraints, and opportunities shaping the industry. It will also delve into regional analysis, competitive landscape, segmentation, and future outlook. Furthermore, the impact of the COVID-19 pandemic on the market and key industry developments will be discussed.
Recombinant vector vaccines are a type of immunization that uses genetically engineered vectors to deliver antigens into the body, stimulating an immune response. These vaccines have gained immense popularity due to their ability to induce strong and long-lasting immune responses. By utilizing vectors such as adenoviruses, lentiviruses, or poxviruses, recombinant vector vaccines can effectively present antigens to the immune system, triggering the production of protective antibodies.
Executive Summary
The Recombinant Vector Vaccine Market is experiencing rapid growth, driven by factors such as the rising prevalence of infectious diseases, increasing investments in vaccine research and development, and advancements in genetic engineering technologies. The market offers immense opportunities for industry participants and stakeholders to capitalize on the growing demand for effective and safe vaccines. However, challenges such as regulatory hurdles and high production costs pose as restraints to market growth. Despite these challenges, the market is expected to witness significant growth in the forecast period.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several key drivers are fueling the growth of the Recombinant Vector Vaccine Market:
Market Restraints
Despite the promising growth prospects, the Recombinant Vector Vaccine Market faces certain challenges:
Market Opportunities
The Recombinant Vector Vaccine Market presents several opportunities for industry participants and stakeholders:
Market Dynamics
The Recombinant Vector Vaccine Market is characterized by dynamic factors that shape its growth trajectory:
Regional Analysis
The Recombinant Vector Vaccine Market exhibits regional variations in terms of market size, growth opportunities, and regulatory landscape. The following regions are key contributors to the market:
Competitive Landscape
Leading Companies in the Recombinant Vector Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
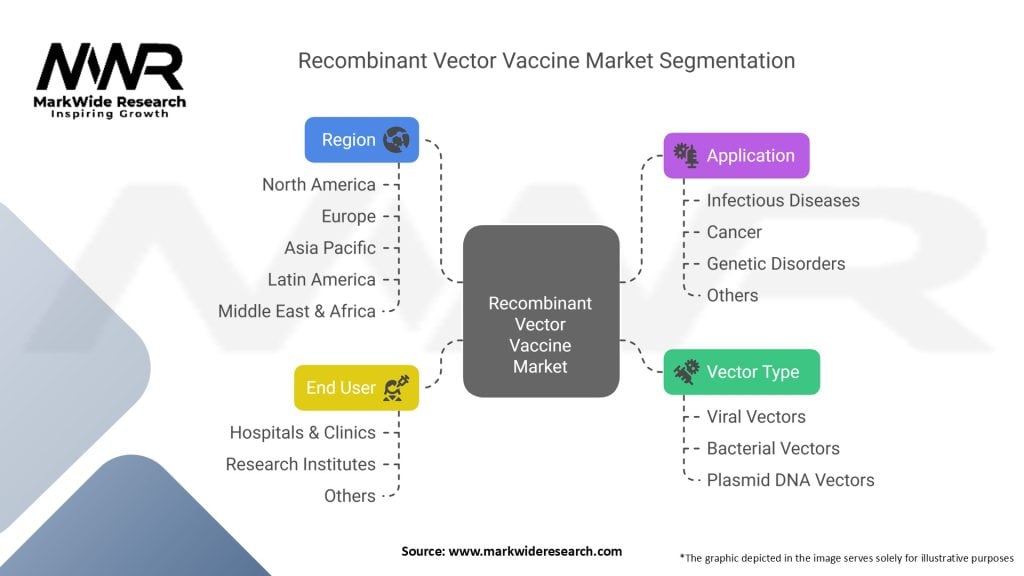
Segmentation
The Recombinant Vector Vaccine Market can be segmented based on various factors, including:
Segmentation allows for a deeper understanding of market dynamics, target audiences, and specific disease areas that present growth opportunities for manufacturers.
Category-wise Insights
The Recombinant Vector Vaccine Market can be further analyzed based on different categories:
Key Benefits for Industry Participants and Stakeholders
The Recombinant Vector Vaccine Market offers several benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats associated with the Recombinant Vector Vaccine Market:
Market Key Trends
Several key trends are shaping the Recombinant Vector Vaccine Market:
COVID-19 Impact
The COVID-19 pandemic has had a profound impact on the Recombinant Vector Vaccine Market. The urgency to develop vaccines against the SARS-CoV-2 virus has accelerated research and development efforts in the field of recombinant vector vaccines. The successful development and authorization of COVID-19 vaccines based on recombinant vector technology have demonstrated the potential of this approach.
The pandemic has also highlighted the importance of vaccine manufacturing capacity, supply chain resilience, and equitable vaccine distribution. Governments, international organizations, and vaccine manufacturers have collaborated to ensure the production, distribution, and administration of COVID-19 vaccines worldwide.
The experience gained from COVID-19 vaccine development and deployment is expected to positively influence the future of recombinant vector vaccines and pave the way for rapid responses to emerging infectious diseases.
Key Industry Developments
The Recombinant Vector Vaccine Market has witnessed several key developments:
Analyst Suggestions
Based on the market analysis, here are some suggestions for industry participants:
Future Outlook
The future of the Recombinant Vector Vaccine Market looks promising, with significant growth potential driven by advancements in genetic engineering, increasing investments in vaccine research and development, and the need for effective preventive healthcare measures. The market is expected to witness the development of novel vaccines targeting a wide range of diseases, including respiratory infections, vector-borne diseases, oncology, and infectious diseases.
The COVID-19 pandemic has highlighted the importance of vaccine development and manufacturing capabilities, as well as the need for global collaborations to address emerging health challenges. Future trends may include the development of personalized vaccines, expansion of multivalent vaccines, and advancements in vector design and delivery systems.
Conclusion
The Recombinant Vector Vaccine Market is experiencing substantial growth, driven by factors such as increasing disease prevalence, technological advancements, and investments in vaccine research and development. While regulatory hurdles and high production costs pose challenges, opportunities lie in emerging markets, product diversification, and collaborations. The COVID-19 pandemic has accelerated vaccine development and highlighted the importance of vaccine manufacturing capacity and equitable distribution. With ongoing advancements and strategic initiatives, the future of the Recombinant Vector Vaccine Market holds immense promise in improving global health outcomes and combating infectious diseases.
What is a recombinant vector vaccine?
A recombinant vector vaccine is a type of vaccine that uses a harmless virus or bacterium as a vector to deliver genetic material from a pathogen, prompting an immune response. This technology is utilized in various applications, including infectious disease prevention and cancer immunotherapy.
What are the key companies in the Recombinant Vector Vaccine Market?
Key companies in the Recombinant Vector Vaccine Market include Merck & Co., Inc., Johnson & Johnson, and AstraZeneca, among others.
What are the drivers of growth in the Recombinant Vector Vaccine Market?
Drivers of growth in the Recombinant Vector Vaccine Market include the increasing prevalence of infectious diseases, advancements in vaccine technology, and rising investments in biotechnology research.
What challenges does the Recombinant Vector Vaccine Market face?
Challenges in the Recombinant Vector Vaccine Market include regulatory hurdles, public skepticism towards vaccines, and the complexity of manufacturing processes.
What opportunities exist in the Recombinant Vector Vaccine Market?
Opportunities in the Recombinant Vector Vaccine Market include the potential for developing vaccines for emerging infectious diseases, expanding applications in personalized medicine, and collaborations between public and private sectors.
What trends are shaping the Recombinant Vector Vaccine Market?
Trends shaping the Recombinant Vector Vaccine Market include the increasing use of mRNA technology, the development of combination vaccines, and a focus on global vaccination initiatives.
Recombinant Vector Vaccine Market
| Segmentation | Details |
|---|---|
| Vector Type | Viral Vectors, Bacterial Vectors, Plasmid DNA Vectors |
| Application | Infectious Diseases, Cancer, Genetic Disorders, Others |
| End User | Hospitals & Clinics, Research Institutes, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Recombinant Vector Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at