444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
Recombinant thrombin is a biotechnological derivative of thrombin, a protein involved in blood clotting. It is widely used in various medical applications, including surgical hemostasis, tissue engineering, drug discovery, and diagnostics. This market overview provides valuable insights into the global recombinant thrombin market, including its meaning, key market insights, drivers, restraints, opportunities, market dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, key benefits for industry participants and stakeholders, SWOT analysis, market key trends, the impact of Covid-19, key industry developments, analyst suggestions, future outlook, and a conclusive summary.
Meaning
Recombinant thrombin refers to a genetically engineered form of thrombin that is produced using biotechnological processes. It is derived from a non-animal source, making it a desirable alternative to thrombin obtained from animal sources, such as bovine or human plasma. The recombinant nature of this thrombin offers several advantages, including enhanced safety, consistency, and availability, making it suitable for a wide range of medical applications.
Executive Summary
The executive summary provides a concise overview of the global recombinant thrombin market, highlighting the key market insights, such as market drivers, restraints, and opportunities. It also summarizes the market dynamics, regional analysis, competitive landscape, and segmentation of the market. This section serves as a quick reference for industry participants and stakeholders to grasp the essential aspects of the market.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
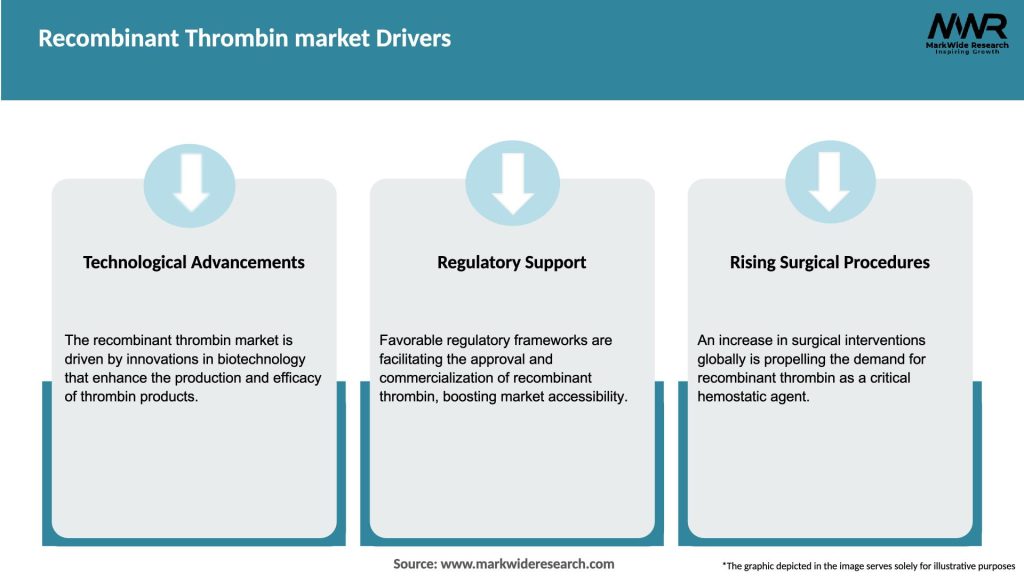
Market Drivers
Market Restraints
Market Opportunities

Market Dynamics
Regional Analysis
The Recombinant Thrombin Market is experiencing strong growth in key regions across the globe. North America and Europe are leading the market, driven by high healthcare spending, advanced medical infrastructure, and the increasing adoption of recombinant thrombin in surgical settings. The US, Germany, and the UK are key markets within these regions, where regulatory approvals and technological advancements have led to the widespread use of recombinant thrombin.
In Asia-Pacific, the market is expanding rapidly due to rising healthcare demands, an increasing number of surgeries, and advancements in biotechnology. China and India are emerging as significant markets, with a growing number of hospitals adopting recombinant thrombin products. Latin America and the Middle East & Africa are expected to see gradual growth as healthcare systems improve, although the market in these regions remains constrained by affordability and infrastructure challenges.
Competitive Landscape
Leading Companies in the Recombinant Thrombin Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
By Component
By Application
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has led to a temporary slowdown in elective surgeries, affecting the demand for recombinant thrombin. However, as healthcare systems recover and surgical procedures resume, the market is expected to see a resurgence. Additionally, the pandemic has increased awareness of the importance of minimizing hospital-acquired infections, further driving the demand for recombinant thrombin.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook section provides a forward-looking perspective on the recombinant thrombin market. It explores anticipated market trends, growth opportunities, and challenges in the coming years. This section enables market players to plan for the future, identify emerging market segments, and align their strategies with the expected market trajectory.
Conclusion
In conclusion, the global recombinant thrombin market presents significant growth opportunitiesin various medical applications. The market overview discussed the meaning of recombinant thrombin and provided an executive summary, key market insights, drivers, restraints, opportunities, market dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, key benefits for industry participants and stakeholders, SWOT analysis, market key trends, the impact of Covid-19, key industry developments, analyst suggestions, future outlook, and a conclusive summary.
Recombinant thrombin offers advantages such as enhanced safety, consistency, and availability compared to thrombin derived from animal sources. The market is driven by factors such as the increasing prevalence of cardiovascular diseases, rising demand for minimally invasive surgical procedures, advancements in biotechnology, and a shift toward recombinant therapeutics. However, stringent regulatory requirements, high production costs, and potential adverse reactions pose challenges to market growth.
What is Recombinant Thrombin?
Recombinant Thrombin is a genetically engineered form of thrombin, an enzyme that plays a crucial role in blood coagulation. It is used in various medical applications, including surgical procedures to promote hemostasis and in wound care to control bleeding.
What are the key players in the Recombinant Thrombin market?
Key players in the Recombinant Thrombin market include companies such as Johnson & Johnson, Baxter International, and Medtronic, which are known for their contributions to hemostatic agents and surgical products, among others.
What are the growth factors driving the Recombinant Thrombin market?
The Recombinant Thrombin market is driven by factors such as the increasing number of surgical procedures, the rising prevalence of bleeding disorders, and advancements in biotechnology that enhance the efficacy of thrombin products.
What challenges does the Recombinant Thrombin market face?
Challenges in the Recombinant Thrombin market include regulatory hurdles for product approval, competition from alternative hemostatic agents, and concerns regarding the safety and efficacy of recombinant products compared to traditional sources.
What opportunities exist in the Recombinant Thrombin market?
Opportunities in the Recombinant Thrombin market include the development of novel formulations, expansion into emerging markets, and increasing demand for minimally invasive surgical techniques that require effective hemostatic agents.
What trends are shaping the Recombinant Thrombin market?
Trends in the Recombinant Thrombin market include the growing focus on personalized medicine, advancements in genetic engineering technologies, and the increasing integration of thrombin products in various surgical specialties, enhancing patient outcomes.
Recombinant Thrombin market
| Segmentation Details | Description |
|---|---|
| Product Type | Topical, Injectable, Lyophilized, Liquid |
| End User | Hospitals, Clinics, Surgical Centers, Research Laboratories |
| Application | Hemostasis, Surgical Procedures, Wound Healing, Tissue Repair |
| Delivery Mode | Intravenous, Subcutaneous, Localized, Systemic |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Recombinant Thrombin Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at