444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Rare Disease Gene Therapy market is a specialized segment within the pharmaceutical and biotechnology industry that focuses on developing therapies for rare genetic diseases. These diseases are characterized by a low prevalence in the population, often affecting fewer than 200,000 individuals in the United States. Gene therapy offers a promising approach to treat these diseases by targeting the underlying genetic defects and providing long-lasting or permanent solutions. The market for rare disease gene therapy is driven by the unmet medical needs of patients suffering from these rare genetic disorders.
Meaning
Rare Disease Gene Therapy refers to the use of genetic engineering techniques to treat or cure rare genetic diseases. It involves the delivery of functional genes or gene-editing tools into a patient’s cells to correct or replace the defective gene responsible for the disease. This innovative approach aims to provide long-term solutions for patients suffering from rare genetic disorders that have no or limited treatment options.
Executive Summary
The Rare Disease Gene Therapy market has witnessed significant advancements in recent years, with the potential to revolutionize the treatment landscape for rare genetic diseases. The market is driven by the increasing understanding of the genetic basis of these diseases, along with advancements in gene delivery technologies and regulatory support for innovative therapies. While the market is still in its early stages, several gene therapy products have been approved and are showing promising results in clinical trials. However, challenges such as high treatment costs and manufacturing complexities need to be addressed for widespread adoption of gene therapies for rare diseases.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several factors are driving the growth of the Rare Disease Gene Therapy market:
Market Restraints
The Rare Disease Gene Therapy market faces certain challenges:
Market Opportunities
Despite the challenges, the Rare Disease Gene Therapy market presents significant opportunities:
Market Dynamics
The Rare Disease Gene Therapy market is dynamic and influenced by various factors:
Regional Analysis
The Rare Disease Gene Therapy market is global in nature, with significant variations in market size and regulatory landscapes across regions. The market is primarily concentrated in developed regions such as North America and Europe, where there is a high prevalence of rare genetic diseases and advanced healthcare systems. These regions also have a robust ecosystem of research institutions, pharmaceutical companies, and regulatory frameworks supporting gene therapy development.
North America, particularly the United States, dominates the market due to factors such as advanced healthcare infrastructure, strong research and development capabilities, and favorable regulatory policies. The region also has a higher prevalence of rare genetic diseases, driving the demand for gene therapies.
Europe is another significant market for Rare Disease Gene Therapy, with countries such as the United Kingdom, Germany, and France at the forefront of gene therapy research and development. The region has well-established regulatory frameworks, including the European Medicines Agency (EMA), which plays a crucial role in facilitating the approval and commercialization of gene therapies.
Asia Pacific is a rapidly emerging market for Rare Disease Gene Therapy, driven by the increasing prevalence of rare genetic diseases, improving healthcare infrastructure, and growing investments in research and development. Countries like China and Japan are investing in the development of gene therapy infrastructure and fostering collaborations with global players.
Latin America and the Middle East & Africa regions are also witnessing growth in the Rare Disease Gene Therapy market, albeit at a slower pace. These regions face challenges related to healthcare infrastructure, access to innovative therapies, and regulatory frameworks. However, initiatives are being taken to address these challenges and foster the development and adoption of gene therapies for rare diseases.
Competitive Landscape
Leading Companies in the Rare Disease Gene Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
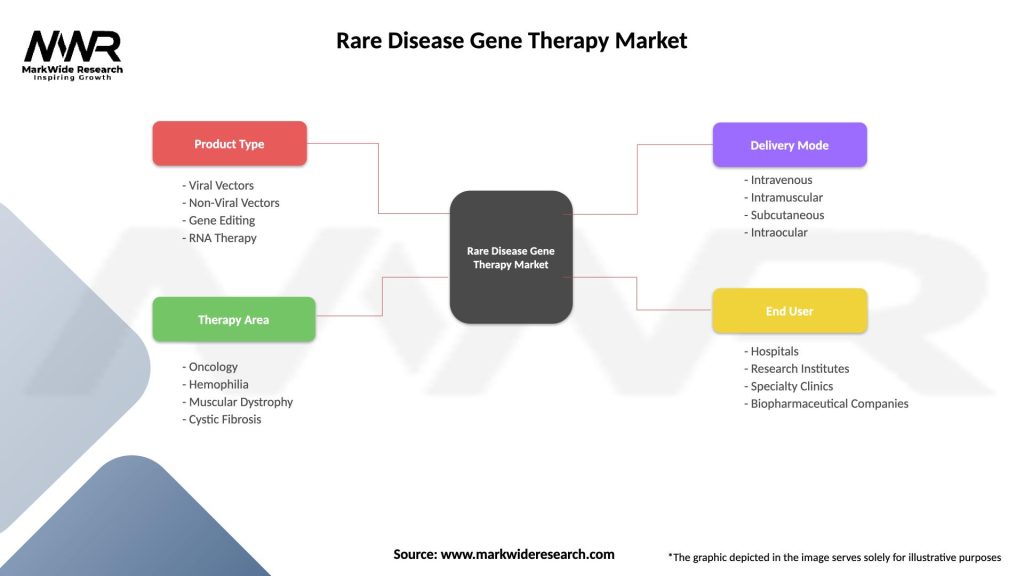
Segmentation
The Rare Disease Gene Therapy market can be segmented based on various factors, including disease type, target gene, delivery system, and region. The following are the key segments:
Segmentation allows for a better understanding of the market landscape, targeted research and development efforts, and tailored marketing strategies.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The Rare Disease Gene Therapy market offers several benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis provides insights into the internal and external factors impacting the Rare Disease Gene Therapy market:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had both positive and negative impacts on the Rare Disease Gene Therapy market. The pandemic brought attention to the vulnerability of rare disease patients and the urgent need for effective treatments. It accelerated regulatory processes and facilitated increased collaboration between industry stakeholders.
However, the pandemic also posed challenges, including disruptions in clinical trials, manufacturing delays, and supply chain disruptions. These challenges impacted the progress of ongoing research and development activities and delayed the commercialization of gene therapies.
Overall, the pandemic underscored the importance of innovative therapies for rare diseases and highlighted the need for resilient manufacturing processes and supply chains to ensure the availability of gene therapies in times of crisis.
Key Industry Developments
Analyst Suggestions
Based on the analysis of the Rare Disease Gene Therapy market, industry analysts provide the following suggestions:
Future Outlook
The future of the Rare Disease Gene Therapy market looks promising, with significant opportunities for the development of innovative treatments for rare genetic diseases. Advancements in gene editing technologies, expanding regulatory support, and increasing investments in research and development are expected to drive the market’s growth.
The market is likely to witness an expansion of the pipeline of gene therapies targeting a wide range of rare diseases. Continued technological advancements, including improvements in gene delivery systems and manufacturing processes, will contribute to enhanced safety, efficacy, and scalability.
However, challenges such as affordability, reimbursement, and long-term safety monitoring need to be addressed for the widespread adoption of gene therapies. Collaboration among stakeholders, including industry, academia, regulatory authorities, and patient advocacy groups, will play a crucial role in overcoming these challenges and ensuring the successful development and accessibility of rare disease gene therapies.
Conclusion
The Rare Disease Gene Therapy market represents a transformative approach to treating rare genetic diseases by targeting the underlying genetic defects. The market offers significant opportunities to address unmet medical needs and provide potential cures or long-term treatments for patients suffering from rare genetic disorders. Technological advancements, regulatory support, and increasing investments are driving the growth of the market.
While challenges exist, including high treatment costs, safety concerns, and manufacturing complexities, the industry is making significant progress in overcoming these barriers. Continued collaboration, research and development efforts, and a patient-centric focus will be crucial to realizing the full potential of Rare Disease Gene Therapy and improving the lives of patients affected by rare genetic diseases.
What is Rare Disease Gene Therapy?
Rare Disease Gene Therapy refers to innovative treatments that use genetic material to address rare genetic disorders. These therapies aim to correct or replace defective genes, providing potential cures for conditions that currently have limited treatment options.
What are the key players in the Rare Disease Gene Therapy Market?
Key players in the Rare Disease Gene Therapy Market include companies like Novartis, Spark Therapeutics, and Bluebird Bio, which are actively developing and commercializing gene therapies for rare diseases, among others.
What are the main drivers of growth in the Rare Disease Gene Therapy Market?
The growth of the Rare Disease Gene Therapy Market is driven by advancements in genetic research, increasing investment in biotechnology, and a growing understanding of genetic disorders. Additionally, regulatory support for expedited approvals enhances market potential.
What challenges does the Rare Disease Gene Therapy Market face?
The Rare Disease Gene Therapy Market faces challenges such as high development costs, complex regulatory pathways, and limited patient populations for clinical trials. These factors can hinder the pace of innovation and market entry for new therapies.
What opportunities exist in the Rare Disease Gene Therapy Market?
Opportunities in the Rare Disease Gene Therapy Market include the potential for personalized medicine approaches, collaborations between biotech firms and academic institutions, and the expansion of gene editing technologies. These factors can lead to the development of novel therapies.
What trends are shaping the Rare Disease Gene Therapy Market?
Trends in the Rare Disease Gene Therapy Market include the rise of CRISPR technology, increased focus on patient-centric approaches, and the growing importance of real-world evidence in therapy development. These trends are influencing how therapies are designed and evaluated.
Rare Disease Gene Therapy Market
| Segmentation Details | Description |
|---|---|
| Product Type | Viral Vectors, Non-Viral Vectors, Gene Editing, RNA Therapy |
| Therapy Area | Oncology, Hemophilia, Muscular Dystrophy, Cystic Fibrosis |
| Delivery Mode | Intravenous, Intramuscular, Subcutaneous, Intraocular |
| End User | Hospitals, Research Institutes, Specialty Clinics, Biopharmaceutical Companies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Rare Disease Gene Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at