444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The pharmaceutical industry is constantly evolving, with new advancements and discoveries being made every day. One important aspect of pharmaceutical development is the analysis of thermal properties of various drug compounds and formulations. Pharmaceutical thermal analysis plays a crucial role in ensuring the quality, stability, and effectiveness of pharmaceutical products. It involves the study of how drugs and drug delivery systems behave under different temperature conditions. This helps in understanding their physical and chemical properties, which in turn aids in formulation design, process optimization, and quality control.
Meaning
Pharmaceutical thermal analysis refers to the scientific investigation of the thermal behavior of drugs and pharmaceutical formulations. It encompasses a range of techniques and methods used to analyze the physical and chemical changes that occur in pharmaceutical compounds when exposed to different temperatures. The goal is to understand the thermal stability, phase transitions, degradation mechanisms, and compatibility of drugs with various excipients and packaging materials. This knowledge is vital for ensuring the safety, efficacy, and shelf-life of pharmaceutical products.
Executive Summary
The pharmaceutical thermal analysis market is witnessing significant growth due to the increasing demand for high-quality drugs and the need for stringent regulatory compliance. This market encompasses various techniques such as differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA), and hot-stage microscopy (HSM). These techniques provide valuable insights into the thermal properties and behavior of pharmaceutical compounds, enabling manufacturers to optimize their formulations and processes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The pharmaceutical thermal analysis market is dynamic and influenced by various factors. Technological advancements, regulatory guidelines, market trends, and the demand for quality pharmaceuticals all shape the market dynamics. The industry is continuously evolving, with new techniques and methodologies being developed to address the challenges faced in drug development and manufacturing. The market dynamics are driven by a need for quality control, regulatory compliance, and advancements in thermal analysis instruments and software. The growing demand for outsourcing services, the rise of personalized medicine, and the emergence of new market opportunities also contribute to the market dynamics.
Regional Analysis
The pharmaceutical thermal analysis market is geographically segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. North America holds a significant share of the market due to the presence of major pharmaceutical companies and research institutions in the region. Europe follows closely, driven by strict regulatory guidelines and a strong emphasis on quality control. The Asia-Pacific region is experiencing rapid growth, attributed to the expanding pharmaceutical industry in countries such as China and India. Latin America, the Middle East, and Africa are also emerging markets with increasing demand for pharmaceutical thermal analysis services.
Competitive Landscape
Leading Companies in Pharmaceutical Thermal Analysis Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
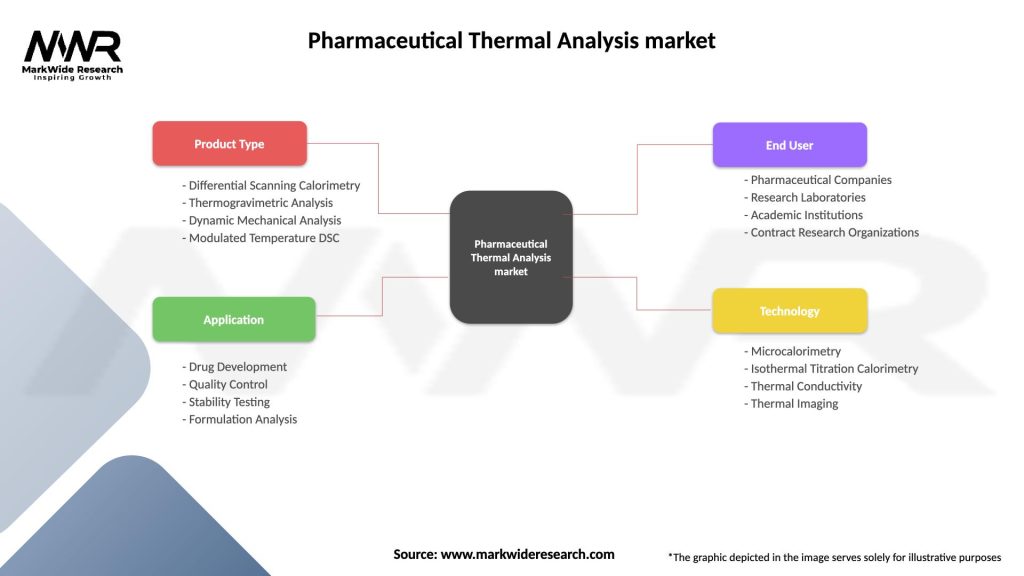
The pharmaceutical thermal analysis market can be segmented based on technique, end user, and region.
By Technique:
By End User:
By Region:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic had a significant impact on the pharmaceutical industry, including the thermal analysis market. The unprecedented global demand for vaccines and therapeutics led to accelerated drug development and manufacturing processes. Thermal analysis played a vital role in ensuring the stability, quality, and efficacy of these products.
Pharmaceutical companies and research institutions utilized thermal analysis techniques to evaluate the thermal stability of vaccine candidates and monitor the impact of different storage and transportation conditions. The need for rapid development and production of COVID-19 vaccines highlighted the importance of thermal analysis in expedited formulation optimization and stability testing.
The pandemic also influenced the market dynamics by increasing the demand for contract research and manufacturing services. Contract research organizations and contract development and manufacturing organizations leveraged thermal analysis techniques to support COVID-19-related research and production activities.
Overall, the COVID-19 pandemic emphasized the critical role of pharmaceutical thermal analysis in drug development, quality control, and ensuring the timely availability of safe and effective vaccines and therapeutics.
Key Industry Developments
Analyst Suggestions
1. Increase Awareness: To further promote the adoption of pharmaceutical thermal analysis, efforts should be made to increase awareness among pharmaceutical manufacturers, researchers, and regulatory bodies. Educational initiatives, workshops, and conferences can help in disseminating knowledge about the benefits and applications of thermal analysis.
Future Outlook
The future of the pharmaceutical thermal analysis market looks promising, driven by increasing demands for quality control, regulatory compliance, and advancements in thermal analysis technology. As the pharmaceutical industry continues to expand, the need for reliable analytical techniques to ensure the stability and efficacy of drugs will grow.
The market is expected to witness further technological advancements, including more sophisticated instruments with higher sensitivity and automation capabilities. These advancements will lead to increased efficiency, accuracy, and throughput in thermal analysis.
The integration of thermal analysis with other analytical techniques and the development of comprehensive data management systems will provide a more holistic understanding of pharmaceutical compounds and formulations.
Additionally, the focus on personalized medicine and the rise of biopharmaceuticals and novel drug delivery systems will create new opportunities for thermal analysis applications.
However, challenges such as high initial investment, lack of skilled personnel, and complex data interpretation need to be addressed. Efforts should be made to overcome these challenges through collaborations, training programs, and the development of cost-effective solutions.
Conclusion
In conclusion, the pharmaceutical thermal analysis market is poised for significant growth, driven by the need for quality control, regulatory compliance, and technological advancements. The market will continue to evolve, providing valuable insights into the thermal behavior of drug compounds and facilitating the development of safe, effective, and innovative pharmaceutical products.
What is Pharmaceutical Thermal Analysis?
Pharmaceutical Thermal Analysis refers to a set of techniques used to study the thermal properties of pharmaceutical substances. This includes methods like differential scanning calorimetry and thermogravimetric analysis, which help in understanding stability, formulation, and compatibility of drugs.
What are the key players in the Pharmaceutical Thermal Analysis market?
Key players in the Pharmaceutical Thermal Analysis market include PerkinElmer, TA Instruments, and Mettler Toledo, among others. These companies provide advanced thermal analysis equipment and solutions tailored for pharmaceutical applications.
What are the growth factors driving the Pharmaceutical Thermal Analysis market?
The Pharmaceutical Thermal Analysis market is driven by the increasing demand for drug development and formulation studies. Additionally, the rise in research activities and the need for quality control in pharmaceuticals contribute to market growth.
What challenges does the Pharmaceutical Thermal Analysis market face?
Challenges in the Pharmaceutical Thermal Analysis market include the high cost of advanced analytical instruments and the need for skilled personnel to operate them. Furthermore, regulatory compliance can complicate the analysis processes.
What opportunities exist in the Pharmaceutical Thermal Analysis market?
Opportunities in the Pharmaceutical Thermal Analysis market include the development of innovative thermal analysis techniques and the expansion of applications in biopharmaceuticals. The growing focus on personalized medicine also presents new avenues for thermal analysis.
What trends are shaping the Pharmaceutical Thermal Analysis market?
Current trends in the Pharmaceutical Thermal Analysis market include the integration of automation and software solutions for data analysis. Additionally, there is a growing emphasis on sustainability and eco-friendly practices in thermal analysis methodologies.
Pharmaceutical Thermal Analysis market
| Segmentation Details | Description |
|---|---|
| Product Type | Differential Scanning Calorimetry, Thermogravimetric Analysis, Dynamic Mechanical Analysis, Modulated Temperature DSC |
| Application | Drug Development, Quality Control, Stability Testing, Formulation Analysis |
| End User | Pharmaceutical Companies, Research Laboratories, Academic Institutions, Contract Research Organizations |
| Technology | Microcalorimetry, Isothermal Titration Calorimetry, Thermal Conductivity, Thermal Imaging |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Pharmaceutical Thermal Analysis Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at