444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Patent Ductus Arteriosus (PDA) Occluder market is witnessing significant growth globally, driven by the increasing prevalence of congenital heart defects, rising demand for minimally invasive treatment options, and technological advancements in interventional cardiology. PDA occluders are medical devices used to close the patent ductus arteriosus, a common congenital heart defect characterized by the persistence of a fetal blood vessel connecting the aorta and pulmonary artery. These devices are deployed through minimally invasive procedures, such as cardiac catheterization, and help restore normal blood flow and cardiac function. With the growing adoption of PDA closure procedures and the expansion of pediatric cardiology services, the demand for PDA occluders is expected to continue rising, driving market expansion and innovation in the coming years.
Meaning
Patent Ductus Arteriosus (PDA) Occluders are medical devices designed to close the patent ductus arteriosus, a congenital heart defect that affects the connection between the aorta and pulmonary artery. PDA occluders are deployed through minimally invasive procedures, such as cardiac catheterization, and help restore normal blood flow and cardiac function by sealing off the abnormal blood vessel. These devices are available in various designs and sizes to accommodate different patient anatomies and procedural requirements. PDA occluders play a crucial role in treating PDA and preventing associated complications, such as heart failure, pulmonary hypertension, and infective endocarditis.
Executive Summary
The global Patent Ductus Arteriosus (PDA) Occluder market is experiencing significant growth, driven by factors such as the increasing prevalence of congenital heart defects, rising demand for minimally invasive treatment options, and technological advancements in interventional cardiology. Key market players are focusing on product innovation, quality assurance, and regulatory compliance to meet the growing demand for PDA occluders and address evolving clinical needs. With the expanding application of PDA closure procedures in pediatric and adult patients, the market is poised for continued expansion and development in the foreseeable future.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Patent Ductus Arteriosus (PDA) Occluder market is characterized by significant growth, technological innovation, and evolving clinical practices in interventional cardiology. Key market players are focusing on research and development, product innovation, and strategic partnerships to gain a competitive edge and capture market share. Additionally, advancements in imaging modalities, device delivery systems, and procedural techniques are driving market expansion and adoption across diverse patient populations and healthcare settings.
Regional Analysis
The Patent Ductus Arteriosus (PDA) Occluder market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America and Europe are the leading markets, driven by the presence of well-established pediatric cardiology services, high prevalence of congenital heart defects, and advanced interventional cardiology programs. Asia Pacific is expected to witness significant growth, fueled by rising healthcare expenditures, increasing adoption of minimally invasive procedures, and expanding access to pediatric cardiology services in emerging economies.
Competitive Landscape
Leading Companies in Patent Ductus Arteriosus (PDA) Occluder Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Patent Ductus Arteriosus (PDA) Occluder market can be segmented based on device type, device design, patient age, and geography. By device type, offerings include coil devices, umbrella devices, plug devices, and others. By device design, designs include detachable devices, retrievable devices, and permanent devices. By patient age, procedures are performed in pediatric patients, adult patients, or both, depending on clinical indications and procedural requirements.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has underscored the importance of minimally invasive treatment options, such as transcatheter PDA closure, in reducing procedural risks and conserving healthcare resources. While the pandemic initially led to disruptions in healthcare services and procedural volumes, it also highlighted the resilience of interventional cardiology programs and the adaptability of healthcare providers in delivering essential cardiac care. As healthcare systems recover and adapt to new challenges, the demand for PDA occluders is expected to rebound, driving market growth and innovation in the post-pandemic era.
Key Industry Developments
Analyst Suggestions
Future Outlook
The global Patent Ductus Arteriosus (PDA) Occluder market is poised for continued growth and innovation in the coming years, driven by increasing demand for minimally invasive treatment options, rising prevalence of congenital heart defects, and technological advancements in interventional cardiology. Key market players are expected to continue investing in research and development, product innovation, and strategic partnerships to meet the growing demand for PDA occluders and address evolving clinical needs. With ongoing advancements in imaging modalities, device delivery systems, and procedural techniques, PDA occluders are poised to remain essential components of pediatric and adult cardiology programs, driving market expansion and adoption across diverse patient populations and healthcare settings.
Conclusion
In conclusion, the Patent Ductus Arteriosus (PDA) Occluder market is witnessing significant growth globally, driven by increasing demand for minimally invasive treatment options, rising prevalence of congenital heart defects, and technological advancements in interventional cardiology. PDA occluders play a crucial role in closing the patent ductus arteriosus and restoring normal blood flow and cardiac function in pediatric and adult patients. Key market players are focusing on research and development, product innovation, and strategic partnerships to expand their market presence and meet the evolving needs of healthcare providers worldwide. With the expanding application of PDA closure procedures and the rise of pediatric and adult cardiology programs, the market is poised for continued expansion and development in the foreseeable future, offering opportunities for innovation, growth, and market leadership.
What is Patent Ductus Arteriosus (PDA) Occluder?
Patent Ductus Arteriosus (PDA) Occluder is a medical device used to close a patent ductus arteriosus, which is a heart defect that occurs when the ductus arteriosus fails to close after birth. This device helps restore normal blood flow and prevent complications associated with the condition.
What are the key players in the Patent Ductus Arteriosus (PDA) Occluder Market?
Key players in the Patent Ductus Arteriosus (PDA) Occluder Market include Abbott Laboratories, Boston Scientific Corporation, and Medtronic, among others. These companies are involved in the development and manufacturing of innovative occluder devices to treat PDA.
What are the growth factors driving the Patent Ductus Arteriosus (PDA) Occluder Market?
The growth of the Patent Ductus Arteriosus (PDA) Occluder Market is driven by increasing prevalence of congenital heart defects, advancements in minimally invasive surgical techniques, and rising awareness about PDA treatment options. These factors contribute to a growing demand for effective occluder devices.
What challenges does the Patent Ductus Arteriosus (PDA) Occluder Market face?
The Patent Ductus Arteriosus (PDA) Occluder Market faces challenges such as stringent regulatory requirements, potential complications associated with the procedure, and the need for skilled healthcare professionals. These factors can hinder market growth and adoption.
What opportunities exist in the Patent Ductus Arteriosus (PDA) Occluder Market?
Opportunities in the Patent Ductus Arteriosus (PDA) Occluder Market include the development of new and improved occluder designs, expansion into emerging markets, and increasing collaborations between medical device companies and healthcare providers. These factors can enhance market potential.
What trends are shaping the Patent Ductus Arteriosus (PDA) Occluder Market?
Trends shaping the Patent Ductus Arteriosus (PDA) Occluder Market include the rise of bioresorbable occluders, advancements in imaging technologies for better procedural outcomes, and a focus on patient-centered care. These trends are influencing product development and market strategies.
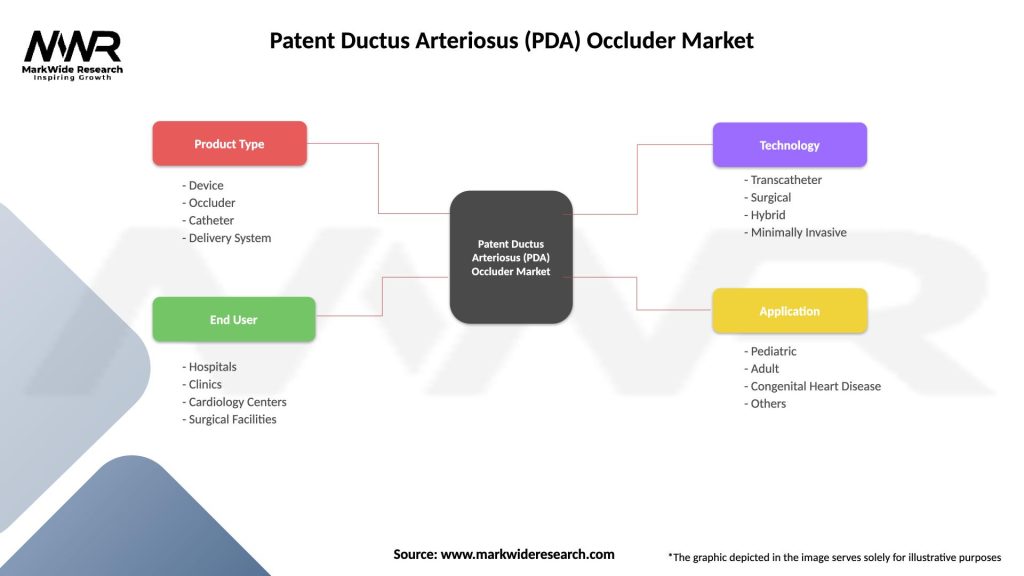
Patent Ductus Arteriosus (PDA) Occluder Market
| Segmentation Details | Description |
|---|---|
| Product Type | Device, Occluder, Catheter, Delivery System |
| End User | Hospitals, Clinics, Cardiology Centers, Surgical Facilities |
| Technology | Transcatheter, Surgical, Hybrid, Minimally Invasive |
| Application | Pediatric, Adult, Congenital Heart Disease, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Patent Ductus Arteriosus (PDA) Occluder Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at