444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The PARP Inhibitor Biomarkers Testing Product Market focuses on diagnostic tools and assays used to identify biomarkers associated with the effectiveness of PARP (Poly ADP-Ribose Polymerase) inhibitors. These inhibitors are a class of cancer drugs that target cancer cells deficient in DNA repair mechanisms, particularly those with BRCA1 and BRCA2 mutations. The market is driven by the increasing adoption of precision medicine in oncology, the rising incidence of cancers with BRCA mutations, and advancements in genomic technologies.
Meaning
PARP inhibitor biomarkers testing products are diagnostic tools designed to detect genetic and molecular biomarkers that predict the responsiveness of cancers to PARP inhibitor therapies. These biomarkers, primarily involving BRCA1 and BRCA2 gene mutations, help identify patients who are most likely to benefit from PARP inhibitors. These tests are essential for personalizing cancer treatment, improving patient outcomes, and optimizing therapeutic strategies.
Executive Summary
The PARP Inhibitor Biomarkers Testing Product Market is experiencing significant growth due to the expanding use of PARP inhibitors in cancer treatment, particularly for ovarian, breast, and prostate cancers. The market is characterized by the development of advanced genomic assays, increasing investments in cancer research, and the growing emphasis on personalized medicine. Key players in the market are focusing on enhancing the sensitivity and specificity of their tests, obtaining regulatory approvals, and forming strategic partnerships to expand their market presence. The future of this market looks promising with ongoing innovations and a broader application of PARP inhibitors in oncology.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The PARP Inhibitor Biomarkers Testing Product Market is influenced by various dynamic factors, including technological advancements, regulatory changes, market competition, and evolving healthcare practices. Companies must navigate these dynamics by continuously innovating, adhering to regulatory standards, and understanding market needs to remain competitive and capture growth opportunities.
Regional Analysis
Competitive Landscape
Leading Companies in the PARP Inhibitor Biomarkers Testing Product Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
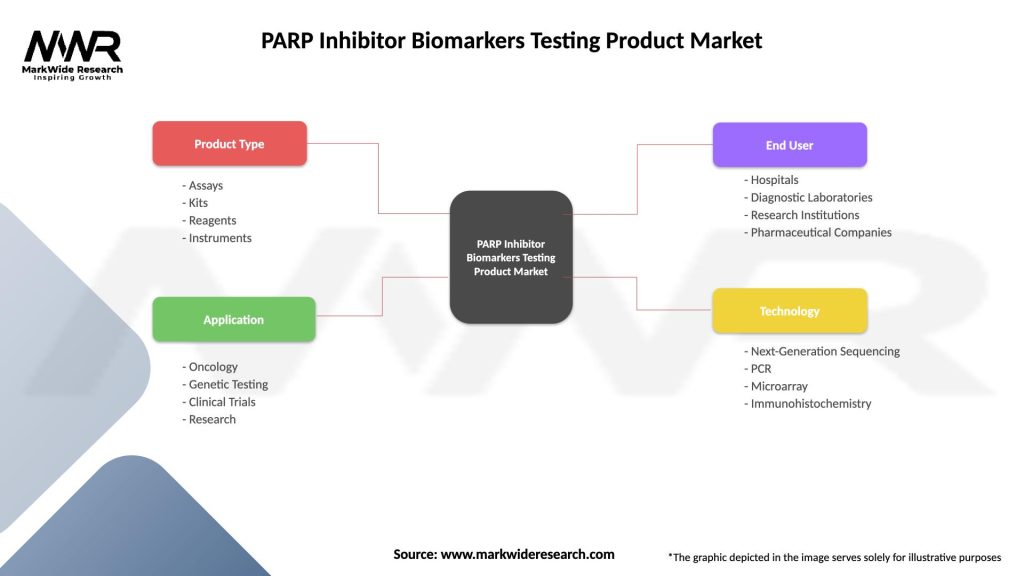
Segmentation
The market can be segmented based on product type, technology, application, end-user, and region:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has impacted the PARP Inhibitor Biomarkers Testing Product Market in several ways:
Key Industry Developments
Analyst Suggestions
Future Outlook
The PARP Inhibitor Biomarkers Testing Product Market is poised for robust growth, driven by technological advancements, increasing prevalence of BRCA-related cancers, and the rising adoption of precision medicine. Market players will continue to innovate and adapt to emerging trends, focusing on patient-centric solutions, regulatory compliance, and market expansion to capture new opportunities and strengthen their market position.
Conclusion
The PARP Inhibitor Biomarkers Testing Product Market is essential for advancing cancer diagnostics and personalized treatment strategies. Technological advancements, rising cancer prevalence, and the shift towards precision medicine are driving market growth and innovation. By investing in product development, expanding market reach, and focusing on patient needs, industry participants can contribute to improved healthcare outcomes and address the evolving challenges in cancer treatment.
What is PARP Inhibitor Biomarkers Testing Product?
PARP Inhibitor Biomarkers Testing Product refers to diagnostic tools and assays used to identify specific biomarkers that indicate the effectiveness of PARP inhibitors in treating certain cancers, particularly breast and ovarian cancers.
What are the key players in the PARP Inhibitor Biomarkers Testing Product Market?
Key players in the PARP Inhibitor Biomarkers Testing Product Market include Myriad Genetics, AstraZeneca, and Roche, among others. These companies are involved in developing innovative testing solutions to enhance personalized medicine approaches in oncology.
What are the growth factors driving the PARP Inhibitor Biomarkers Testing Product Market?
The growth of the PARP Inhibitor Biomarkers Testing Product Market is driven by the increasing prevalence of cancer, advancements in genomic testing technologies, and the rising demand for personalized treatment options in oncology.
What challenges does the PARP Inhibitor Biomarkers Testing Product Market face?
Challenges in the PARP Inhibitor Biomarkers Testing Product Market include regulatory hurdles, the complexity of biomarker identification, and the need for extensive clinical validation to ensure the accuracy and reliability of tests.
What future opportunities exist in the PARP Inhibitor Biomarkers Testing Product Market?
Future opportunities in the PARP Inhibitor Biomarkers Testing Product Market include the development of novel biomarkers, integration of artificial intelligence in testing processes, and expansion into emerging markets with growing healthcare infrastructure.
What trends are shaping the PARP Inhibitor Biomarkers Testing Product Market?
Trends shaping the PARP Inhibitor Biomarkers Testing Product Market include the increasing focus on companion diagnostics, the rise of liquid biopsy technologies, and the growing collaboration between pharmaceutical companies and diagnostic firms.
PARP Inhibitor Biomarkers Testing Product Market
| Segmentation Details | Description |
|---|---|
| Product Type | Assays, Kits, Reagents, Instruments |
| Application | Oncology, Genetic Testing, Clinical Trials, Research |
| End User | Hospitals, Diagnostic Laboratories, Research Institutions, Pharmaceutical Companies |
| Technology | Next-Generation Sequencing, PCR, Microarray, Immunohistochemistry |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the PARP Inhibitor Biomarkers Testing Product Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at