444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Oral Anticoagulant Reversal Drug market addresses the critical need for medications that can swiftly reverse the effects of oral anticoagulants in emergency situations. These drugs are essential for managing bleeding complications or preparing patients for urgent surgical interventions. The market is driven by factors such as the increasing use of oral anticoagulants, rising prevalence of cardiovascular diseases, and growing demand for antidotes that can effectively reverse anticoagulant effects.
Meaning
Oral anticoagulant reversal drugs are pharmacological agents used to counteract the anticoagulant effects of medications such as warfarin, direct oral anticoagulants (DOACs), and other blood-thinning agents. These reversal agents work by neutralizing the anticoagulant activity of oral anticoagulants, restoring normal blood clotting function, and mitigating the risk of bleeding complications. Rapid reversal of anticoagulation is crucial in emergency situations, such as acute bleeding events or the need for urgent surgical procedures, to prevent adverse outcomes and improve patient safety.
Executive Summary
The Oral Anticoagulant Reversal Drug market is witnessing significant growth, driven by the expanding use of oral anticoagulants for the management of thromboembolic disorders and atrial fibrillation. The market benefits from the introduction of novel reversal agents, advancements in antidote development, and regulatory approvals for expanded indications. Key players in the market focus on product innovation, clinical evidence generation, and strategic partnerships to address unmet medical needs and enhance patient care in anticoagulation management.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Oral Anticoagulant Reversal Drug market is influenced by various factors, including disease epidemiology, treatment guidelines, regulatory policies, reimbursement landscape, and market competition. Key market players focus on research and development, clinical evidence generation, market access strategies, and post-market surveillance to address unmet medical needs and maintain competitive advantage. Collaboration among stakeholders, including pharmaceutical companies, regulatory agencies, healthcare providers, and patient advocacy groups, is essential for driving innovation, improving patient outcomes, and advancing anticoagulation management.
Regional Analysis
The Oral Anticoagulant Reversal Drug market is segmented into regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. North America dominates the global market, driven by factors such as advanced healthcare infrastructure, high prevalence of cardiovascular diseases, and regulatory approvals for reversal agents. Europe follows closely, supported by robust regulatory frameworks, clinical expertise, and market access pathways. Asia-Pacific and Latin America present significant growth opportunities due to expanding anticoagulant utilization, increasing awareness of reversal strategies, and improving healthcare access.
Competitive Landscape
Leading Companies in Oral Anticoagulant Reversal Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
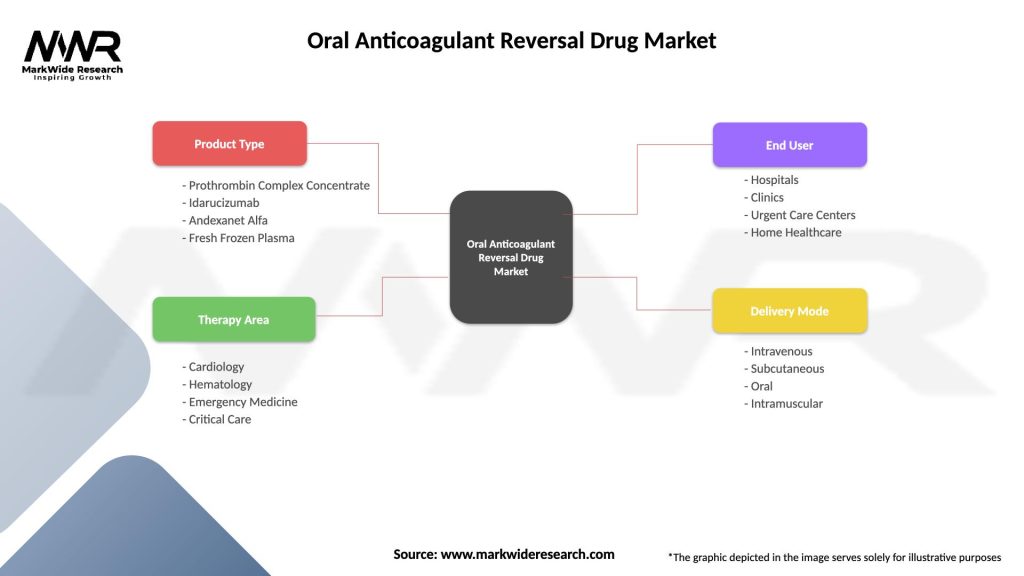
Segmentation
The Oral Anticoagulant Reversal Drug market can be segmented based on antidote type, anticoagulant class, clinical indication, end-user, and region. Antidote types include specific reversal agents for vitamin K antagonists, direct thrombin inhibitors, and factor Xa inhibitors, as well as non-specific hemostatic agents for broad-spectrum reversal activity. Anticoagulant classes encompass warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, and other emerging oral anticoagulants. Clinical indications for reversal agents include acute bleeding events, urgent surgical interventions, and overdose situations requiring rapid anticoagulation reversal.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has impacted the Oral Anticoagulant Reversal Drug market, leading to changes in healthcare delivery, anticoagulation management, and patient care. The pandemic has heightened awareness of thrombotic complications associated with COVID-19 infection, prompting increased utilization of oral anticoagulants for thromboprophylaxis in hospitalized patients and those at high risk of venous thromboembolism. As anticoagulant use rises, so does the demand for reversal agents to manage bleeding events and ensure patient safety. Despite challenges such as supply chain disruptions, healthcare resource constraints, and changes in treatment protocols, the market for oral anticoagulant reversal drugs has demonstrated resilience, driven by the critical need for effective reversal strategies in anticoagulated patients.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Oral Anticoagulant Reversal Drug market is poised for continued growth and innovation in the coming years, driven by factors such as the increasing prevalence of cardiovascular diseases, rising utilization of oral anticoagulants, and advancements in antidote development. Market players must adapt to evolving market dynamics, regulatory requirements, and clinical practice guidelines by investing in research and development, evidence generation, and market access strategies. Collaboration among stakeholders, including pharmaceutical companies, healthcare providers, regulatory agencies, and patient advocacy groups, is essential for driving innovation, improving patient outcomes, and advancing anticoagulation management globally.
Conclusion
In conclusion, the Oral Anticoagulant Reversal Drug market addresses the critical need for medications that can swiftly reverse the effects of oral anticoagulants in emergency situations, preventing adverse outcomes and improving patient safety. Reversal agents play a crucial role in managing bleeding complications, preparing patients for urgent surgical interventions, and optimizing hemostasis in anticoagulated populations. Despite challenges such as limited clinical evidence, reimbursement barriers, and regulatory hurdles, the market for oral anticoagulant reversal drugs is expected to continue expanding, driven by the increasing use of oral anticoagulants and growing demand for effective reversal strategies. Market players must prioritize innovation, evidence-based practice, and interdisciplinary collaboration to address unmet medical needs and improve patient outcomes in anticoagulation management.
What is Oral Anticoagulant Reversal Drug?
Oral Anticoagulant Reversal Drug refers to medications designed to counteract the effects of oral anticoagulants, which are used to prevent blood clots. These drugs are crucial in emergency situations where rapid reversal of anticoagulation is necessary to prevent excessive bleeding.
What are the key players in the Oral Anticoagulant Reversal Drug Market?
Key players in the Oral Anticoagulant Reversal Drug Market include companies like Boehringer Ingelheim, Portola Pharmaceuticals, and Bristol-Myers Squibb, among others. These companies are involved in the development and commercialization of reversal agents for anticoagulants.
What are the growth factors driving the Oral Anticoagulant Reversal Drug Market?
The growth of the Oral Anticoagulant Reversal Drug Market is driven by the increasing prevalence of conditions requiring anticoagulation therapy, such as atrial fibrillation and venous thromboembolism. Additionally, the rising awareness of the need for effective reversal agents in emergency care contributes to market expansion.
What challenges does the Oral Anticoagulant Reversal Drug Market face?
The Oral Anticoagulant Reversal Drug Market faces challenges such as regulatory hurdles in drug approval processes and the high cost of developing new reversal agents. Furthermore, the potential for adverse effects and the need for careful patient monitoring can complicate their use.
What opportunities exist in the Oral Anticoagulant Reversal Drug Market?
Opportunities in the Oral Anticoagulant Reversal Drug Market include the development of novel reversal agents with improved safety profiles and efficacy. Additionally, expanding applications in surgical settings and emergency medicine present significant growth potential.
What trends are shaping the Oral Anticoagulant Reversal Drug Market?
Trends in the Oral Anticoagulant Reversal Drug Market include the increasing focus on personalized medicine and the integration of technology in drug delivery systems. Moreover, ongoing research into new anticoagulant therapies is likely to influence the development of corresponding reversal agents.
Oral Anticoagulant Reversal Drug Market
| Segmentation Details | Description |
|---|---|
| Product Type | Prothrombin Complex Concentrate, Idarucizumab, Andexanet Alfa, Fresh Frozen Plasma |
| Therapy Area | Cardiology, Hematology, Emergency Medicine, Critical Care |
| End User | Hospitals, Clinics, Urgent Care Centers, Home Healthcare |
| Delivery Mode | Intravenous, Subcutaneous, Oral, Intramuscular |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Oral Anticoagulant Reversal Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at