444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The nucleic acid-based diagnostic market stands at the forefront of molecular diagnostics, offering precise and rapid detection of various diseases through the analysis of nucleic acids. This market segment encompasses a diverse array of diagnostic techniques, including PCR, DNA sequencing, and microarrays, which play pivotal roles in identifying genetic disorders, infectious diseases, and cancer biomarkers.
Meaning
Nucleic acid-based diagnostics entail the detection and analysis of genetic material, such as DNA or RNA, to diagnose diseases and disorders. Leveraging molecular techniques, these diagnostics offer unparalleled specificity and sensitivity, enabling early disease detection, personalized treatment strategies, and therapeutic monitoring.
Executive Summary
The nucleic acid-based diagnostic market is witnessing exponential growth, driven by advancements in genomic technologies, rising demand for personalized medicine, and the pressing need for accurate diagnostic tools amidst global health challenges. Despite regulatory complexities and cost considerations, the market presents lucrative opportunities for industry players poised to innovate and address unmet clinical needs.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The nucleic acid-based diagnostic market operates within a dynamic landscape shaped by technological innovation, regulatory evolution, healthcare policy reforms, and shifting disease epidemiology. Industry stakeholders must navigate these dynamics adeptly, leveraging market trends, addressing unmet clinical needs, and fostering strategic partnerships to drive sustainable growth and market leadership.
Regional Analysis
Regional variations in the nucleic acid-based diagnostic market stem from differences in healthcare infrastructure, regulatory frameworks, reimbursement policies, and disease prevalence patterns. An in-depth analysis of regional dynamics enables market players to tailor strategies, localize product offerings, and capitalize on emerging opportunities across diverse geographic markets.
Competitive Landscape
Leading Companies in Nucleic Acid-Based Diagnostic Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
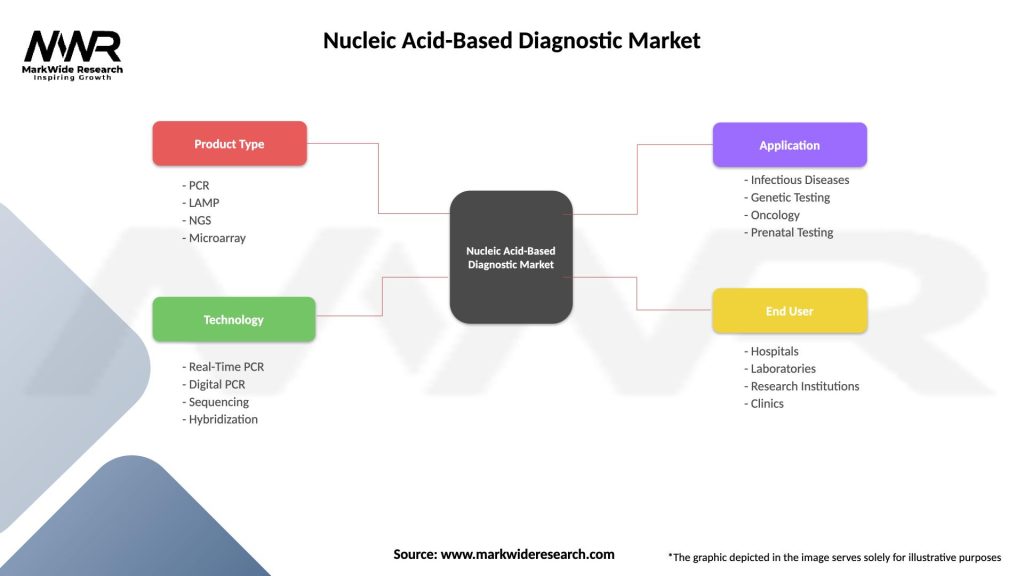
Segmentation
Segmentation of the nucleic acid-based diagnostic market enables targeted product development, market positioning, and customer segmentation, catering to diverse clinical needs, disease indications, and end-user preferences.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Nucleic acid-based diagnostics offer myriad benefits for industry participants and stakeholders, including:
SWOT Analysis
A SWOT analysis of the nucleic acid-based diagnostic market reveals:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has profoundly impacted the nucleic acid-based diagnostic market, accelerating innovation, adoption, and investment in diagnostic technologies. Key impacts include:
Key Industry Developments
Analyst Suggestions
Future Outlook
The nucleic acid-based diagnostic market is poised for robust growth in the post-pandemic era, driven by sustained demand for infectious disease testing, oncology diagnostics, and personalized medicine applications. Key trends such as technological innovation, digital health integration, and regulatory evolution will shape the market landscape, fostering innovation, market expansion, and healthcare transformation.
Conclusion
In conclusion, the nucleic acid-based diagnostic market represents a cornerstone of modern healthcare, offering unparalleled diagnostic capabilities, personalized treatment strategies, and disease surveillance solutions. Despite regulatory challenges, cost considerations, and technological complexities, the market presents abundant opportunities for industry stakeholders to innovate, collaborate, and address unmet clinical needs. By embracing emerging technologies, fostering strategic partnerships, and navigating regulatory pathways adeptly, nucleic acid-based diagnostic companies can drive sustainable growth, advance precision medicine initiatives, and contribute meaningfully to global health outcomes.
What is Nucleic Acid-Based Diagnostic?
Nucleic Acid-Based Diagnostics refer to techniques that utilize nucleic acids, such as DNA and RNA, to detect and diagnose diseases. These methods are widely used in molecular biology and clinical diagnostics for identifying pathogens, genetic disorders, and cancers.
What are the key players in the Nucleic Acid-Based Diagnostic Market?
Key players in the Nucleic Acid-Based Diagnostic Market include Thermo Fisher Scientific, Roche Diagnostics, and Abbott Laboratories, among others. These companies are known for their innovative diagnostic solutions and extensive product portfolios.
What are the growth factors driving the Nucleic Acid-Based Diagnostic Market?
The growth of the Nucleic Acid-Based Diagnostic Market is driven by the increasing prevalence of infectious diseases, advancements in genomic technologies, and the rising demand for personalized medicine. Additionally, the need for rapid and accurate diagnostic tools is propelling market expansion.
What challenges does the Nucleic Acid-Based Diagnostic Market face?
The Nucleic Acid-Based Diagnostic Market faces challenges such as high costs associated with advanced diagnostic technologies and regulatory hurdles in obtaining approvals. Furthermore, the complexity of some tests can limit their accessibility in certain healthcare settings.
What opportunities exist in the Nucleic Acid-Based Diagnostic Market?
Opportunities in the Nucleic Acid-Based Diagnostic Market include the development of point-of-care testing solutions and the integration of artificial intelligence in diagnostics. These innovations can enhance testing efficiency and expand access to diagnostic services.
What trends are shaping the Nucleic Acid-Based Diagnostic Market?
Trends in the Nucleic Acid-Based Diagnostic Market include the increasing adoption of next-generation sequencing technologies and the growing focus on companion diagnostics. These trends are enhancing the precision of diagnostics and supporting the development of targeted therapies.
Nucleic Acid-Based Diagnostic Market
| Segmentation Details | Description |
|---|---|
| Product Type | PCR, LAMP, NGS, Microarray |
| Technology | Real-Time PCR, Digital PCR, Sequencing, Hybridization |
| Application | Infectious Diseases, Genetic Testing, Oncology, Prenatal Testing |
| End User | Hospitals, Laboratories, Research Institutions, Clinics |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Nucleic Acid-Based Diagnostic Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at