444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The North America Transdermal Drug Delivery Systems (TDDS) market is witnessing significant growth due to the rising prevalence of chronic diseases, increasing demand for non-invasive drug delivery methods, and advancements in transdermal technology. TDDS offer several advantages over conventional oral and injectable drug delivery systems, including improved patient compliance, controlled drug release, and reduced systemic side effects. With the growing emphasis on personalized medicine and targeted therapies, the North America TDDS market is poised for substantial expansion in the coming years.
Meaning
Transdermal drug delivery involves the administration of medications through the skin for systemic absorption into the bloodstream. Transdermal patches, gels, creams, and films are examples of TDDS that deliver drugs directly through the skin, bypassing the gastrointestinal tract and avoiding first-pass metabolism. This route of drug administration offers advantages such as sustained release, reduced dosing frequency, and improved patient adherence, making it a preferred choice for delivering a wide range of therapeutics, including analgesics, hormone replacement therapies, and nicotine replacement products.
Executive Summary
The North America TDDS market is experiencing rapid growth driven by factors such as the increasing prevalence of chronic diseases, rising demand for targeted drug delivery solutions, and technological advancements in transdermal drug delivery systems. Market players are focusing on innovation, product development, and strategic collaborations to capitalize on emerging opportunities and gain a competitive edge in the market. However, regulatory challenges, patent expirations, and pricing pressures pose significant challenges to market growth. Understanding key market trends, drivers, and restraints is essential for stakeholders to navigate the evolving landscape and capitalize on growth opportunities in the North America TDDS market.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The North America TDDS market operates in a dynamic environment shaped by factors such as technological innovation, regulatory trends, competitive dynamics, and shifting consumer preferences. These dynamics influence market trends, growth trajectories, and investment strategies for industry participants and stakeholders. Understanding the market dynamics is essential for companies to identify opportunities, mitigate risks, and capitalize on emerging trends in the North America TDDS market.
Regional Analysis
The North America TDDS market is characterized by a mature regulatory landscape, robust healthcare infrastructure, and strong market demand for innovative drug delivery solutions. The United States dominates the market, accounting for the majority of TDDS sales and revenue in the region. Factors such as favorable reimbursement policies, advanced research capabilities, and strategic collaborations with academic institutions and research organizations drive market growth and innovation in North America.
Competitive Landscape
Leading Companies in North America Transdermal Drug Delivery Systems Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The North America TDDS market can be segmented based on various factors such as product type, application, distribution channel, and geography. Common product types include transdermal patches, gels, creams, and films, which are used to deliver drugs for indications such as pain management, hormone replacement therapy, and smoking cessation. Applications of TDDS include dermatology, neurology, cardiovascular diseases, and oncology, among others. Distribution channels for TDDS include retail pharmacies, hospital pharmacies, online pharmacies, and specialty clinics.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has accelerated the adoption of transdermal drug delivery systems in North America, driven by factors such as the need for remote patient monitoring, reduced healthcare facility visits, and enhanced infection control measures. Some key impacts of COVID-19 on the North America TDDS market include:
Key Industry Developments
Analyst Suggestions
Future Outlook
The North America TDDS market is poised for significant growth in the coming years, driven by factors such as technological innovation, expanding therapeutic applications, and increasing patient demand for non-invasive drug delivery solutions. However, challenges such as regulatory complexities, patent expirations, and pricing pressures will require industry players to adopt proactive strategies, leverage partnerships, and invest in innovation to sustain growth and competitiveness in the dynamic healthcare landscape of North America.
Conclusion
The North America Transdermal Drug Delivery Systems (TDDS) market offers significant growth opportunities driven by factors such as the rising prevalence of chronic diseases, technological advancements in drug delivery technology, and increasing demand for personalized medicine. Companies operating in this market must navigate regulatory challenges, address market needs, and leverage partnerships to capitalize on emerging opportunities and gain a competitive edge. By focusing on innovation, collaboration, and patient-centric design, industry players can contribute to advancing healthcare delivery and improving patient outcomes in North America.
What is Transdermal Drug Delivery Systems?
Transdermal Drug Delivery Systems are methods of delivering medication through the skin for systemic effects. These systems are designed to provide controlled release of drugs, improving patient compliance and minimizing side effects.
What are the key players in the North America Transdermal Drug Delivery Systems Market?
Key players in the North America Transdermal Drug Delivery Systems Market include Johnson & Johnson, Novartis, and Mylan, among others. These companies are involved in the development and commercialization of various transdermal products.
What are the growth factors driving the North America Transdermal Drug Delivery Systems Market?
The growth of the North America Transdermal Drug Delivery Systems Market is driven by factors such as the increasing prevalence of chronic diseases, the demand for pain management solutions, and advancements in drug formulation technologies.
What challenges does the North America Transdermal Drug Delivery Systems Market face?
Challenges in the North America Transdermal Drug Delivery Systems Market include skin irritation issues, limited drug types suitable for transdermal delivery, and regulatory hurdles that can delay product approvals.
What opportunities exist in the North America Transdermal Drug Delivery Systems Market?
Opportunities in the North America Transdermal Drug Delivery Systems Market include the development of new formulations for vaccines and hormones, as well as the potential for personalized medicine approaches that enhance drug delivery efficiency.
What trends are shaping the North America Transdermal Drug Delivery Systems Market?
Trends in the North America Transdermal Drug Delivery Systems Market include the increasing use of microneedle technology, the rise of wearable drug delivery devices, and a growing focus on patient-centric drug delivery solutions.
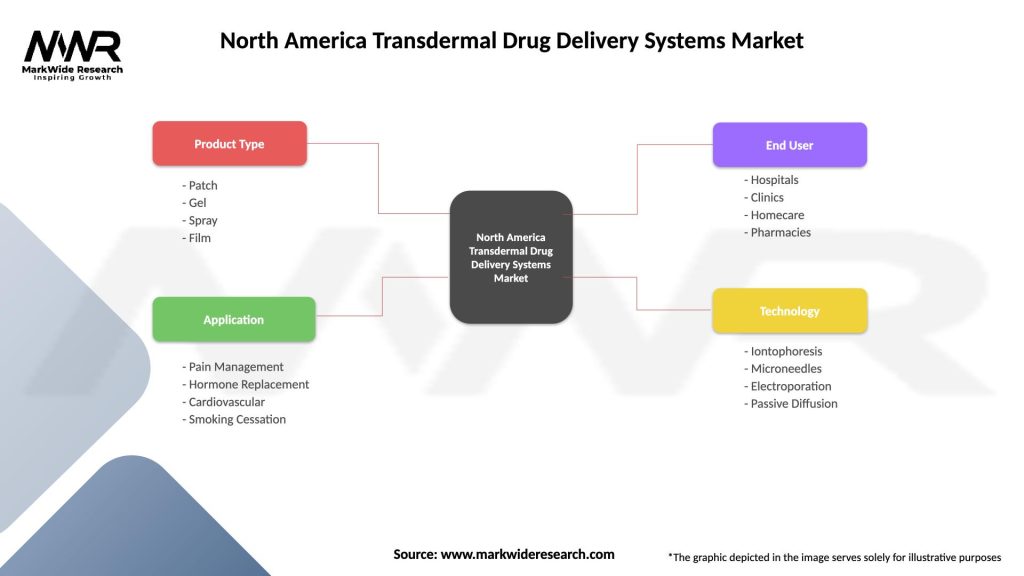
North America Transdermal Drug Delivery Systems Market
| Segmentation Details | Description |
|---|---|
| Product Type | Patch, Gel, Spray, Film |
| Application | Pain Management, Hormone Replacement, Cardiovascular, Smoking Cessation |
| End User | Hospitals, Clinics, Homecare, Pharmacies |
| Technology | Iontophoresis, Microneedles, Electroporation, Passive Diffusion |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in North America Transdermal Drug Delivery Systems Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at