444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview:
The North America Ovarian Cancer Diagnostics market is a critical segment within the healthcare industry, dedicated to diagnosing and detecting ovarian cancer in patients. Ovarian cancer is a prevalent and potentially life-threatening disease that affects women, making early and accurate diagnosis crucial for effective treatment. The market encompasses various diagnostic tools and techniques, including imaging tests, blood tests, biopsies, and genetic testing. With a focus on improving early detection rates and enhancing diagnostic accuracy, the North America Ovarian Cancer Diagnostics market plays a vital role in improving patient outcomes and reducing the burden of ovarian cancer in the region.
Meaning:
The North America Ovarian Cancer Diagnostics market refers to the healthcare industry’s efforts to identify and diagnose ovarian cancer in women. The market involves the use of various diagnostic tests and tools to detect cancerous cells and tumors in the ovaries, allowing healthcare professionals to devise appropriate treatment plans for patients.
Executive Summary:
The North America Ovarian Cancer Diagnostics market has experienced significant advancements in recent years, driven by factors such as increased awareness of ovarian cancer, technological innovations in diagnostic techniques, and a growing emphasis on early detection. Ovarian cancer remains a significant health concern for women, and the market’s focus on improving diagnostic accuracy and efficiency is crucial in improving patient outcomes. With a commitment to research, development, and collaboration, the market offers significant opportunities for industry participants to make a positive impact on women’s health in North America.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
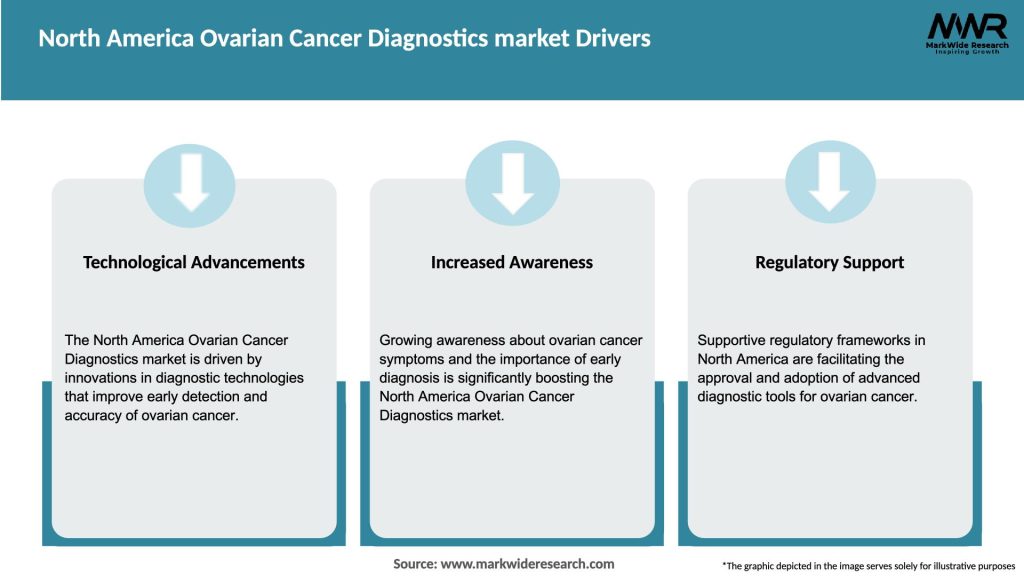
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The North America Ovarian Cancer Diagnostics market operates in a dynamic healthcare landscape, influenced by advancements in medical technology, changing disease trends, and evolving patient needs. Industry participants must adapt to shifting market dynamics and invest in research and innovation to improve diagnostic capabilities and patient outcomes.
Regional Analysis:
The North America Ovarian Cancer Diagnostics market can be segmented into regions, including the United States, Canada, Mexico, and other countries within North America. The United States represents the largest market within the region, owing to its well-established healthcare infrastructure and extensive research initiatives.
Competitive Landscape:
Leading Companies in North America Ovarian Cancer Diagnostics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
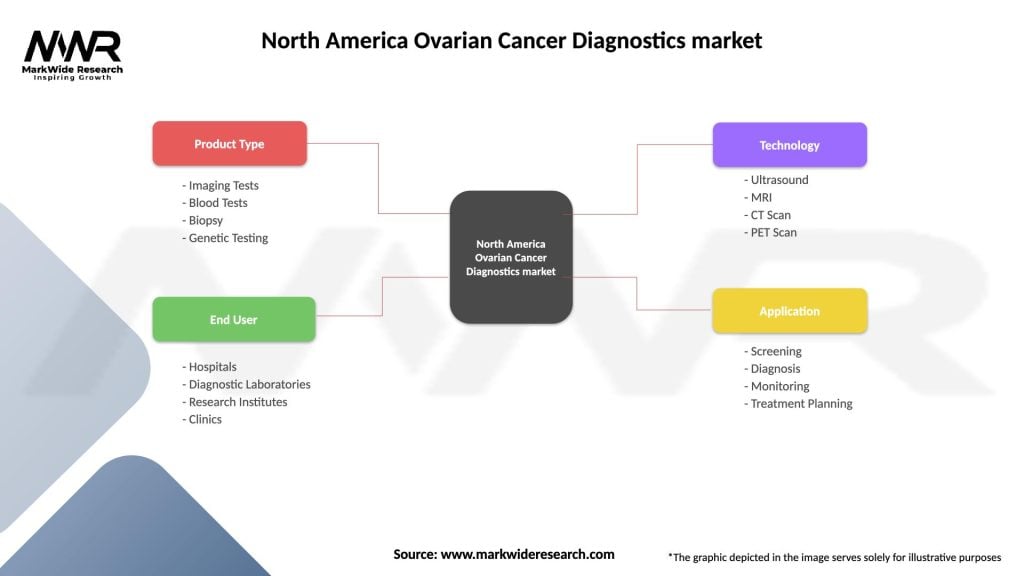
Segmentation:
The North America Ovarian Cancer Diagnostics market can be segmented based on various factors, including diagnostic techniques, end-users, and applications. Additionally, segmentation based on geographical regions can provide valuable insights into regional prevalence and healthcare disparities.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic had varying impacts on the North America Ovarian Cancer Diagnostics market. While the initial period saw disruptions in healthcare services and diagnostics, the market witnessed a focus on telemedicine solutions and remote patient monitoring to address patient needs during the pandemic.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The North America Ovarian Cancer Diagnostics market is expected to witness continued growth in the coming years, driven by the rising incidence of ovarian cancer and the focus on early detection. As industry players prioritize research, innovation, and patient-centric care, the market offers ample opportunities to improve patient outcomes and reduce the burden of ovarian cancer in the region.
Conclusion:
The North America Ovarian Cancer Diagnostics market plays a pivotal role in detecting and diagnosing ovarian cancer in women. With a focus on early detection, accuracy, and personalized medicine, the market’s efforts contribute to improved patient outcomes and better women’s health. As industry participants invest in research, technology integration, and collaborative efforts, the future outlook for the North America Ovarian Cancer Diagnostics market remains promising. Industry stakeholders must continue to prioritize advancements in diagnostics and patient care to make a positive impact on ovarian cancer management and treatment in the region.
What is Ovarian Cancer Diagnostics?
Ovarian Cancer Diagnostics refers to the methods and technologies used to detect and diagnose ovarian cancer, including imaging techniques, blood tests, and biopsy procedures.
What are the key players in the North America Ovarian Cancer Diagnostics market?
Key players in the North America Ovarian Cancer Diagnostics market include Roche, Abbott Laboratories, and Thermo Fisher Scientific, among others.
What are the growth factors driving the North America Ovarian Cancer Diagnostics market?
The growth of the North America Ovarian Cancer Diagnostics market is driven by increasing awareness of ovarian cancer, advancements in diagnostic technologies, and a rise in the aging population.
What challenges does the North America Ovarian Cancer Diagnostics market face?
Challenges in the North America Ovarian Cancer Diagnostics market include high costs of advanced diagnostic tests, regulatory hurdles, and the need for skilled professionals to interpret results.
What opportunities exist in the North America Ovarian Cancer Diagnostics market?
Opportunities in the North America Ovarian Cancer Diagnostics market include the development of novel biomarkers for early detection, increasing investment in research and development, and the potential for personalized medicine approaches.
What trends are shaping the North America Ovarian Cancer Diagnostics market?
Trends in the North America Ovarian Cancer Diagnostics market include the integration of artificial intelligence in diagnostic processes, the rise of liquid biopsy technologies, and a focus on patient-centered care.
North America Ovarian Cancer Diagnostics market
| Segmentation Details | Description |
|---|---|
| Product Type | Imaging Tests, Blood Tests, Biopsy, Genetic Testing |
| End User | Hospitals, Diagnostic Laboratories, Research Institutes, Clinics |
| Technology | Ultrasound, MRI, CT Scan, PET Scan |
| Application | Screening, Diagnosis, Monitoring, Treatment Planning |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in North America Ovarian Cancer Diagnostics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at