444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The non-PVC IV bags market is experiencing significant growth due to the increasing demand for safer and eco-friendly intravenous fluid packaging solutions. Intravenous (IV) bags are widely used in healthcare settings to administer fluids, medications, and nutrition to patients. Traditional IV bags are typically made of polyvinyl chloride (PVC), which contains harmful chemicals such as phthalates that can leach into the fluids and pose risks to patient health. In recent years, there has been a growing concern about the use of PVC IV bags, leading to the development and adoption of non-PVC alternatives.
Meaning
Non-PVC IV bags refer to intravenous fluid containers that are made from materials other than polyvinyl chloride. These bags are designed to provide a safer and more environmentally friendly solution for intravenous fluid administration. Non-PVC IV bags are free from phthalates and other harmful chemicals found in PVC bags, reducing the risk of contamination and adverse health effects for patients. These bags are available in various sizes and configurations to meet the diverse needs of healthcare facilities and patients.
Executive Summary
The non-PVC IV bags market is witnessing steady growth, driven by the rising demand for safer and more sustainable intravenous fluid packaging solutions. Healthcare providers and regulatory bodies are increasingly recognizing the potential health risks associated with PVC IV bags and are shifting towards non-PVC alternatives. The market is characterized by the presence of several key players offering a wide range of non-PVC IV bags. Regional analysis reveals significant growth opportunities in emerging economies. However, market growth may be hindered by certain challenges such as high manufacturing costs and limited awareness among healthcare professionals.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The non-PVC IV bags market is dynamic, with several factors influencing its growth and development. The market is driven by the increasing awareness of health hazards associated with PVC IV bags, along with stringent regulatory initiatives promoting the adoption of safer alternatives. Environmental concerns and advancements in material technology further contribute to market growth. However, challenges such as high manufacturing costs, limited awareness, supply chain disruptions, and compatibility issues can hinder the market’s expansion. Opportunities lie in emerging economies, technological advancements, collaborations, and rising patient demand for safer intravenous fluid packaging solutions. Continued innovation and strategic partnerships are crucial for sustained growth in the non-PVC IV bags market.
Regional Analysis
The non-PVC IV bags market is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. Each region has its own set of factors influencing market growth.
Competitive Landscape
Leading companies in the Non-PVC IV Bags Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
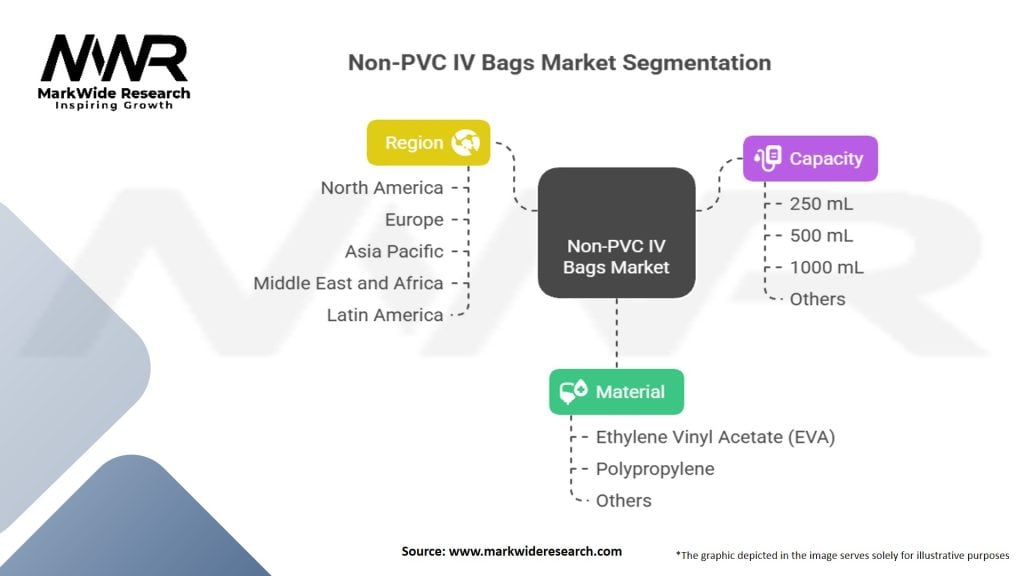
The non-PVC IV bags market can be segmented based on material type, capacity, end-user, and region.
The segmentation allows for a better understanding of market trends, preferences, and regional dynamics, helping industry participants make informed decisions regarding product development, marketing strategies, and market expansion.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the non-PVC IV bags market can benefit in several ways:
SWOT Analysis
A SWOT analysis of the non-PVC IV bags market provides insights into the strengths, weaknesses, opportunities, and threats that impact the market’s growth and competitiveness.
Understanding these internal and external factors helps industry participants formulate effective strategies to leverage strengths, address weaknesses, capitalize on opportunities, and mitigate potential threats in the non-PVC IV bags market.
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the healthcare industry, including the non-PVC IV bags market. The increased demand for intravenous fluid administration during the pandemic has highlighted the importance of safe and reliable IV bag solutions. Healthcare facilities have become more conscious of infection control measures and the potential risks associated with PVC IV bags.
The pandemic has also disrupted global supply chains, causing temporary shortages of medical supplies, including PVC IV bags. This situation has further emphasized the need for diversified and reliable sources of non-PVC IV bags to ensure uninterrupted patient care.
Additionally, the Covid-19 pandemic has accelerated the adoption of telemedicine and homecare settings, where the use of non-PVC IV bags can play a crucial role in providing safe and convenient intravenous fluid administration outside of traditional healthcare facilities.
Overall, the Covid-19 pandemic has underscored the importance of non-PVC IV bags in ensuring patient safety, infection control, and the resilience of healthcare systems.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the non-PVC IV bags market is promising. The increasing awareness of health hazards associated with PVC IV bags, coupled with stringent regulatory initiatives, will continue to drive the adoption of non-PVC alternatives. Technological advancements and ongoing research in material science will lead to the development of safer, more functional, and cost-effective non-PVC IV bag solutions.
Market growth opportunities in emerging economies, rising patient demand for safer healthcare practices, and the growing focus on sustainability will further propel the market forward. Collaboration between industry participants, healthcare providers, and regulatory bodies will be crucial to address challenges, drive innovation, and ensure the safe and effective use of non-PVC IV bags.
Conclusion
The non-PVC IV bags market is witnessing significant growth as healthcare facilities and regulatory bodies recognize the importance of safer and more environmentally friendly intravenous fluid packaging solutions. The shift away from PVC IV bags is driven by concerns about health hazards, such as phthalate leaching, and the need for sustainable practices in the healthcare sector.
However, challenges such as high manufacturing costs, limited awareness, supply chain disruptions, and compatibility issues need to be addressed. To thrive in the market, industry participants should focus on raising awareness, enhancing product development, strengthening distribution networks, embracing sustainability, and ensuring regulatory compliance.
What are Non-PVC IV Bags?
Non-PVC IV Bags are intravenous bags made from materials other than polyvinyl chloride, often utilizing alternatives like polyethylene or ethylene vinyl acetate. These bags are designed to reduce the risk of leaching harmful chemicals into the fluids they contain, making them safer for patient use.
Who are the key players in the Non-PVC IV Bags Market?
Key players in the Non-PVC IV Bags Market include Baxter International, B. Braun Melsungen AG, and Fresenius Kabi, among others. These companies are known for their innovative approaches and extensive product lines in the medical supplies sector.
What are the growth factors driving the Non-PVC IV Bags Market?
The growth of the Non-PVC IV Bags Market is driven by increasing awareness of the health risks associated with PVC materials, a rising demand for safer medical products, and the expansion of healthcare facilities globally. Additionally, the shift towards environmentally friendly packaging solutions is contributing to market growth.
What challenges does the Non-PVC IV Bags Market face?
The Non-PVC IV Bags Market faces challenges such as higher production costs compared to traditional PVC bags and potential supply chain issues related to sourcing alternative materials. Additionally, regulatory hurdles can impact the speed of product development and market entry.
What opportunities exist in the Non-PVC IV Bags Market?
Opportunities in the Non-PVC IV Bags Market include the development of new materials that enhance bag performance and safety, as well as the potential for expansion into emerging markets where healthcare infrastructure is improving. Innovations in manufacturing processes can also lead to cost reductions and increased adoption.
What trends are shaping the Non-PVC IV Bags Market?
Trends in the Non-PVC IV Bags Market include a growing preference for sustainable and eco-friendly medical products, advancements in material science leading to better bag performance, and increased regulatory scrutiny on medical packaging. These trends are influencing both consumer choices and manufacturer strategies.
Non-PVC IV Bags Market
| Segmentation | Details |
|---|---|
| Material | Ethylene Vinyl Acetate (EVA), Polypropylene, Others |
| Capacity | 250 mL, 500 mL, 1000 mL, Others |
| Region | North America, Europe, Asia Pacific, Middle East and Africa, Latin America |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Non-PVC IV Bags Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at