444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The MRI Safe Defibrillator market is experiencing significant growth and innovation in the field of cardiac care. As medical technology continues to advance, the demand for safe and efficient defibrillators compatible with magnetic resonance imaging (MRI) has surged. This market overview aims to provide valuable insights into the MRI Safe Defibrillator market, including its meaning, executive summary, key market insights, drivers, restraints, opportunities, dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, benefits for industry participants and stakeholders, SWOT analysis, key trends, COVID-19 impact, industry developments, analyst suggestions, future outlook, and conclusion.
Meaning
MRI Safe Defibrillators are specialized medical devices designed to provide life-saving electrical shocks to patients experiencing life-threatening cardiac arrhythmias. These defibrillators are uniquely engineered to be safe for use in MRI environments, where the presence of strong magnetic fields requires non-ferromagnetic and MRI-compatible materials. They ensure patient safety during MRI scans by minimizing the risks associated with electromagnetic interference and avoiding potential harm caused by conventional defibrillators in MRI settings.
Executive Summary
The MRI Safe Defibrillator market is witnessing substantial growth due to the rising prevalence of cardiovascular diseases, technological advancements in MRI-compatible defibrillators, and the increasing adoption of MRI scanners in healthcare facilities. The market offers lucrative opportunities for manufacturers, healthcare providers, and stakeholders to meet the growing demand for safe and reliable defibrillation devices in MRI environments. However, certain challenges such as high costs, stringent regulatory requirements, and limited awareness among healthcare professionals may hamper market growth.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The MRI Safe Defibrillator market is characterized by dynamic factors that impact its growth trajectory. Technological advancements, market competition, changing regulatory landscapes, and evolving patient preferences shape the market dynamics. Continuous research and development, strategic collaborations, and market expansion efforts are crucial to capitalize on emerging opportunities and address the evolving needs of patients and healthcare providers.
Regional Analysis
The MRI Safe Defibrillator market exhibits regional variations influenced by factors such as healthcare infrastructure, government initiatives, reimbursement policies, and the prevalence of cardiac diseases. North America currently dominates the market due to the high adoption of advanced medical technologies and well-established healthcare systems. Europe follows closely, driven by a strong emphasis on patient safety and technological advancements. The Asia Pacific region is expected to witness significant growth owing to improving healthcare infrastructure and a growing patient population.
Competitive Landscape
Leading Companies in the MRI Safe Defibrillator Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
The market can be segmented based on product type, end-user, and geography. Product types include external defibrillators, implantable defibrillators, and wearable defibrillators. End-users encompass hospitals, ambulatory surgical centers, and cardiac specialty clinics.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
COVID-19 Impact
The COVID-19 pandemic has had a mixed impact on the MRI Safe Defibrillator market. While the market initially faced disruptions due to supply chain disruptions and a shift in healthcare priorities, the focus on remote monitoring and telemedicine has increased the demand for implantable and wearable defibrillators. Additionally, the emphasis on infection control and patient safety has further propelled the adoption of MRI Safe Defibrillators in healthcare settings.
Key Industry Developments
Key developments in the MRI Safe Defibrillator Market include:
Analyst Suggestions
Future Outlook
The future of the MRI Safe Defibrillator market looks promising, driven by advancements in technology, increasing prevalence of cardiac diseases, and the growing adoption of MRI scanners in healthcare facilities. The market is expected to witness significant growth as manufacturers continue to innovate and develop more efficient and user-friendly MRI-compatible defibrillators. Strategic collaborations and partnerships between key players will further fuel market growth and improve patient outcomes.
Conclusion
The MRI Safe Defibrillator market is poised for remarkable growth as the demand for safe and efficient cardiac care solutions increases. With the integration of advanced technologies, patient safety enhancements, and expanding applications in healthcare settings, MRI Safe Defibrillators are revolutionizing the field of cardiac care. Industry participants, healthcare providers, and stakeholders have a unique opportunity to contribute to this evolving market by embracing innovation, collaboration, and continuous improvement. By addressing challenges, leveraging opportunities, and prioritizing patient-centric solutions, the MRI Safe Defibrillator market will continue to advance and positively impact the lives of cardiac patients worldwide.
What is MRI Safe Defibrillator?
An MRI Safe Defibrillator is a medical device designed to safely deliver electrical shocks to patients experiencing cardiac arrest while they are in an MRI environment. These defibrillators are engineered to be compatible with MRI machines, minimizing the risk of interference or harm during imaging procedures.
What are the key companies in the MRI Safe Defibrillator market?
Key companies in the MRI Safe Defibrillator market include Medtronic, Philips Healthcare, and Zoll Medical Corporation, among others. These companies are known for their innovative technologies and commitment to enhancing patient safety in MRI settings.
What are the growth factors driving the MRI Safe Defibrillator market?
The growth of the MRI Safe Defibrillator market is driven by the increasing prevalence of cardiovascular diseases and the rising number of MRI procedures performed globally. Additionally, advancements in medical technology and a growing focus on patient safety in healthcare facilities contribute to market expansion.
What challenges does the MRI Safe Defibrillator market face?
The MRI Safe Defibrillator market faces challenges such as high manufacturing costs and the need for rigorous regulatory approvals. Furthermore, the limited awareness among healthcare providers regarding the availability and benefits of MRI safe devices can hinder market growth.
What opportunities exist in the MRI Safe Defibrillator market?
Opportunities in the MRI Safe Defibrillator market include the development of advanced defibrillation technologies and the potential for partnerships between medical device manufacturers and healthcare providers. Additionally, increasing investments in healthcare infrastructure present avenues for growth.
What trends are shaping the MRI Safe Defibrillator market?
Trends in the MRI Safe Defibrillator market include the integration of wireless technology for remote monitoring and the development of compact, portable devices. There is also a growing emphasis on training healthcare professionals to effectively use these devices in MRI environments.
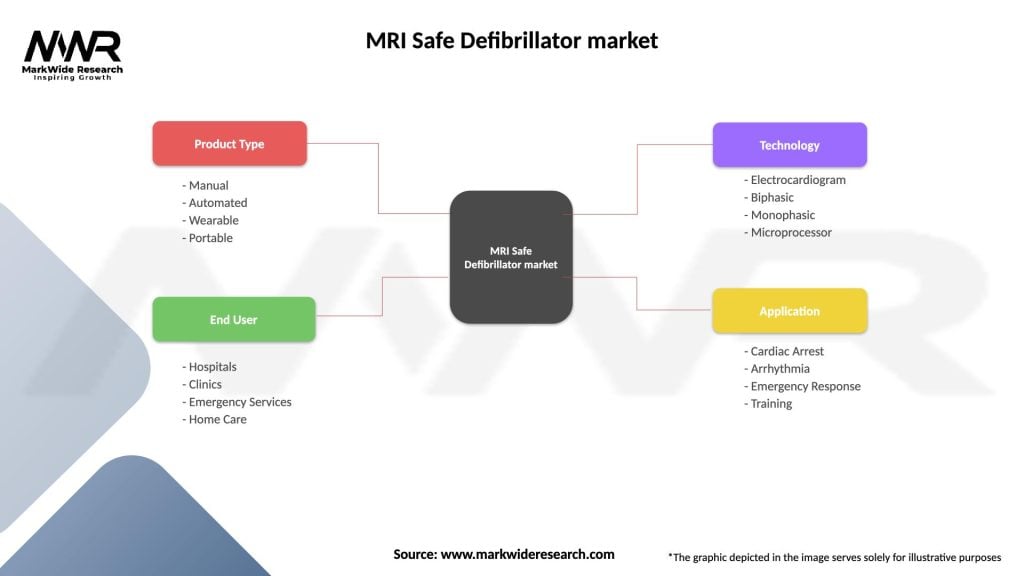
MRI Safe Defibrillator market
| Segmentation Details | Description |
|---|---|
| Product Type | Manual, Automated, Wearable, Portable |
| End User | Hospitals, Clinics, Emergency Services, Home Care |
| Technology | Electrocardiogram, Biphasic, Monophasic, Microprocessor |
| Application | Cardiac Arrest, Arrhythmia, Emergency Response, Training |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the MRI Safe Defibrillator Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at