444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The modular sterile isolator market is a vital segment within the pharmaceutical and biotechnology industries, providing critical containment solutions for aseptic processing, sterile compounding, and handling of hazardous materials. These isolators create a controlled environment that prevents contamination and protects both the product and operators during various manufacturing and research processes.
Meaning
Modular sterile isolators are enclosed systems designed to maintain a sterile environment by controlling parameters such as air quality, pressure differentials, temperature, and humidity. They consist of a sealed chamber with integrated glove ports, access doors, and sterilization systems, allowing operators to perform tasks inside the isolator without exposing the contents to external contaminants.
Executive Summary
The modular sterile isolator market has witnessed significant growth due to the increasing demand for sterile manufacturing environments, stringent regulatory requirements, and the expansion of the biopharmaceutical sector. These isolators offer advantages such as flexibility, scalability, and ease of installation, making them indispensable for pharmaceutical companies, contract manufacturers, and research laboratories worldwide.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The modular sterile isolator market operates in a dynamic environment shaped by technological innovation, regulatory developments, competitive pressures, and evolving customer demands. Manufacturers must adapt to changing market dynamics, anticipate future trends, and align their strategies to capitalize on emerging opportunities and mitigate potential risks.
Regional Analysis
The modular sterile isolator market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory landscape, economic development, and industry trends. While North America and Europe dominate the market due to established pharmaceutical sectors and stringent regulatory standards, Asia Pacific is poised for rapid growth driven by increasing investments in biopharmaceutical manufacturing and research activities.
Competitive Landscape
Leading Companies: Modular Sterile Isolator Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
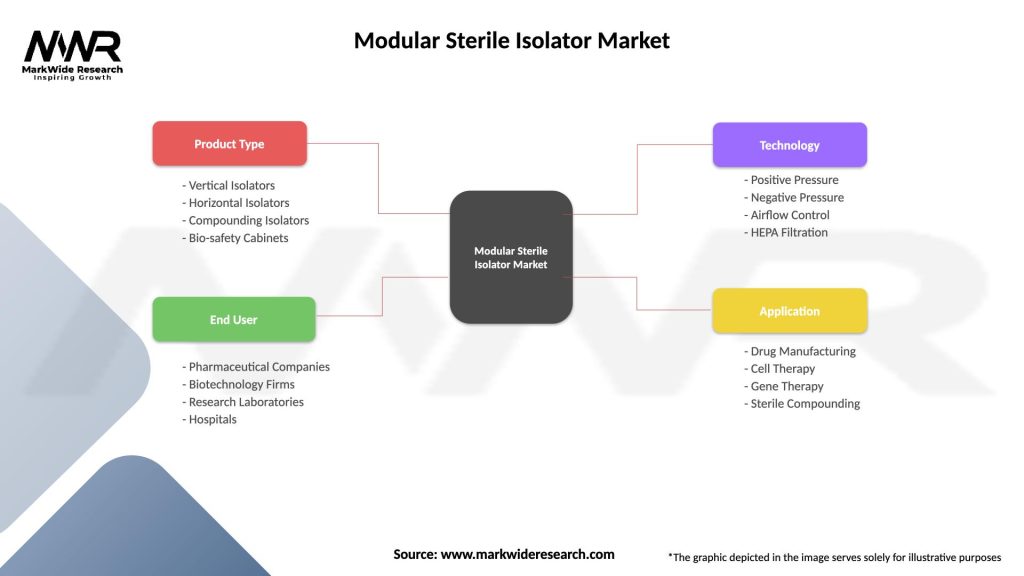
Segmentation
The modular sterile isolator market can be segmented based on:
Segmentation provides insights into market dynamics, customer preferences, and competitive landscapes, enabling companies to develop targeted marketing strategies, optimize product portfolios, and drive revenue growth in specific market segments.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has accelerated the adoption of modular sterile isolators in pharmaceutical manufacturing, vaccine production, and infectious disease research, driven by the need for sterile manufacturing environments, biocontainment facilities, and containment solutions for hazardous pathogens and biological agents.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the modular sterile isolator market is optimistic, driven by factors such as the increasing demand for sterile manufacturing environments, stringent regulatory requirements, technological advancements, and the expanding pharmaceutical and biotechnology sectors. Continued investment in innovation, regulatory compliance, market expansion, and strategic partnerships will be essential for modular sterile isolator manufacturers to capitalize on emerging trends, address evolving customer needs, and sustain long-term growth and competitiveness in the dynamic pharmaceutical manufacturing and bioprocessing markets.
Conclusion
In conclusion, the modular sterile isolator market presents significant opportunities for growth and innovation within the pharmaceutical and biotechnology industries. With the increasing emphasis on sterile manufacturing, stringent regulatory standards, and advancements in biopharmaceutical production, modular sterile isolators have become indispensable for ensuring product quality, operator safety, and regulatory compliance.
Despite challenges such as high initial investment, complex validation processes, and supply chain disruptions, key industry players have the opportunity to leverage emerging trends such as automation, single-use technologies, and smart monitoring systems to drive market expansion and address evolving customer needs.
The COVID-19 pandemic has underscored the importance of modular sterile isolators in pharmaceutical manufacturing, vaccine production, and infectious disease research, driving market demand and adoption. Looking ahead, investment in innovation, regulatory compliance, customer-centricity, and strategic partnerships will be crucial for manufacturers to capitalize on market opportunities, overcome challenges, and sustain long-term growth and competitiveness in the dynamic pharmaceutical manufacturing landscape.
What is Modular Sterile Isolator?
A Modular Sterile Isolator is a controlled environment system designed to provide a sterile workspace for handling sensitive materials, particularly in pharmaceutical and biotechnology applications. These isolators are crucial for maintaining aseptic conditions during processes such as drug formulation and compounding.
What are the key players in the Modular Sterile Isolator Market?
Key players in the Modular Sterile Isolator Market include companies like Getinge AB, STERIS plc, and Merck KGaA, which are known for their innovative solutions in sterile processing and containment technologies. These companies focus on enhancing product safety and compliance in various applications, among others.
What are the growth factors driving the Modular Sterile Isolator Market?
The Modular Sterile Isolator Market is driven by increasing demand for sterile environments in pharmaceutical manufacturing, rising concerns over contamination, and the growth of biologics and biosimilars. Additionally, advancements in isolator technology are enhancing operational efficiency and safety.
What challenges does the Modular Sterile Isolator Market face?
Challenges in the Modular Sterile Isolator Market include high initial investment costs and the complexity of regulatory compliance. Furthermore, the need for skilled personnel to operate and maintain these systems can also pose a barrier to widespread adoption.
What opportunities exist in the Modular Sterile Isolator Market?
Opportunities in the Modular Sterile Isolator Market include the growing trend of personalized medicine and the increasing adoption of advanced manufacturing technologies. The expansion of biopharmaceutical companies also presents significant growth potential for isolator solutions.
What trends are shaping the Modular Sterile Isolator Market?
Trends in the Modular Sterile Isolator Market include the integration of automation and digital technologies to enhance monitoring and control. Additionally, there is a shift towards modular designs that allow for flexibility and scalability in sterile processing environments.
Modular Sterile Isolator Market
| Segmentation Details | Description |
|---|---|
| Product Type | Vertical Isolators, Horizontal Isolators, Compounding Isolators, Bio-safety Cabinets |
| End User | Pharmaceutical Companies, Biotechnology Firms, Research Laboratories, Hospitals |
| Technology | Positive Pressure, Negative Pressure, Airflow Control, HEPA Filtration |
| Application | Drug Manufacturing, Cell Therapy, Gene Therapy, Sterile Compounding |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies: Modular Sterile Isolator Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at