444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Middle East and Africa (MEA) region is witnessing significant advancements in the field of regenerative medicine, particularly in the area of induced pluripotent stem cells (iPSCs). iPSCs are derived from adult cells and possess the remarkable ability to differentiate into various cell types, making them a valuable tool for disease modeling, drug discovery, and personalized medicine. The MEA iPSCs market is experiencing substantial growth as governments and healthcare institutions recognize the potential of this technology to revolutionize medical treatments and improve patient outcomes.
Meaning
Induced pluripotent stem cells (iPSCs) are a type of stem cell that are artificially generated by reprogramming adult cells, such as skin cells or blood cells, back to a pluripotent state. Pluripotent cells have the capacity to differentiate into any cell type in the body, offering immense potential for regenerative medicine and tissue engineering applications. iPSCs have become a focus of intense research and development efforts in the Middle East and Africa region, as scientists and clinicians explore their therapeutic applications in various diseases and disorders.
Executive Summary
The Middle East and Africa induced pluripotent stem cells (iPSCs) market is poised for remarkable growth in the coming years. The region is witnessing increasing investments in research and development activities, as well as collaborations between academic institutions, pharmaceutical companies, and government bodies. These initiatives are aimed at advancing iPSC technology and its clinical applications, thereby driving market growth. The MEA iPSCs market is characterized by a favorable regulatory environment, rising awareness about the potential benefits of iPSC-based therapies, and a growing demand for personalized medicine solutions.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The MEA iPSCs market is characterized by dynamic factors that influence its growth trajectory. These dynamics include technological advancements, evolving regulatory landscapes, changing healthcare priorities, and market competition. The market is expected to witness significant developments driven by ongoing research, strategic collaborations, and increasing investments in infrastructure and talent.
Regional Analysis
The Middle East and Africa region comprises diverse countries with varying levels of healthcare infrastructure and research capabilities. While some countries in the region have made significant progress in stem cell research and regenerative medicine, others are still in the early stages of adoption. The regional analysis of the MEA iPSCs market provides insights into the market dynamics, trends, and opportunities specific to each country or sub-region.
Competitive Landscape
Leading Companies in Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
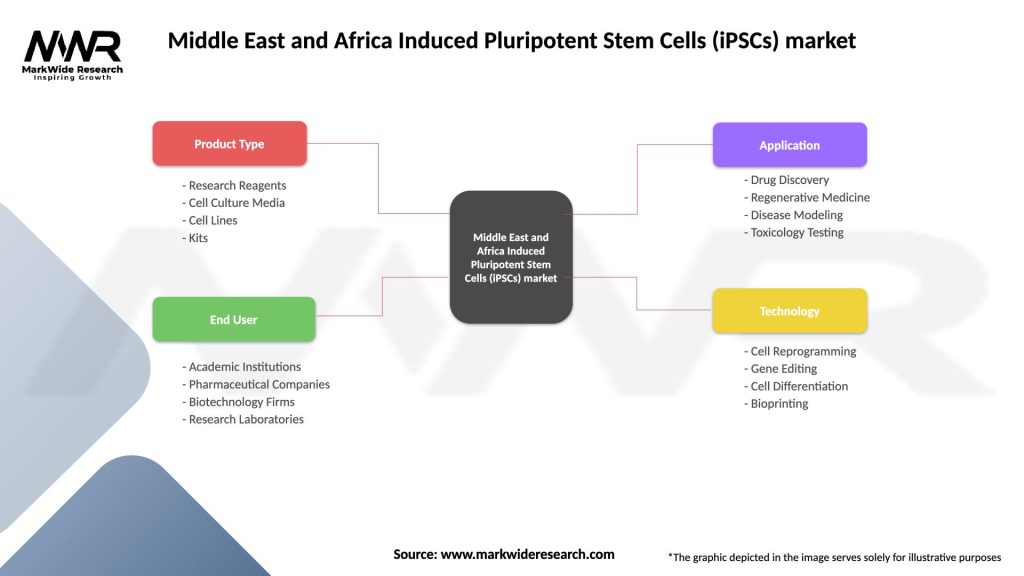
Segmentation
The MEA iPSCs market can be segmented based on:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the MEA iPSCs market. While the pandemic disrupted healthcare systems and research activities, it also highlighted the importance of regenerative medicine and the potential of iPSCs in combating infectious diseases. The pandemic accelerated research efforts in iPSC-based vaccine development, drug screening, and disease modeling, driving advancements in the field. The focus on personalized medicine and the need for innovative therapies to address the long-term effects of COVID-19 further contributed to the growth of the iPSCs market in the region.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the Middle East and Africa iPSCs market is highly promising. The region is poised to witness significant advancements in iPSC research, clinical applications, and commercialization efforts. The convergence of technological advancements, supportive regulatory frameworks, and increasing investments will drive market growth. The MEAiPSCs market is expected to witness the development of novel therapies, expansion of stem cell banking services, and the emergence of personalized medicine approaches. Collaborative research initiatives, strategic partnerships, and advancements in gene editing techniques will further propel the market forward. The COVID-19 pandemic has underscored the importance of regenerative medicine and accelerated the adoption of iPSCs in infectious disease research. With ongoing developments and a favorable market environment, the MEA iPSCs market is poised for a bright future.
Conclusion
The Middle East and Africa induced pluripotent stem cells (iPSCs) market is experiencing significant growth and offers immense potential for the development of regenerative therapies and personalized medicine solutions. iPSCs have emerged as a valuable tool for disease modeling, drug discovery, and tissue engineering applications, driving research and commercialization efforts in the region. Despite challenges such as ethical concerns and high development costs, supportive government initiatives, increasing healthcare expenditure, and collaborative research efforts are propelling market growth. The MEA region is witnessing advancements in stem cell research, expanding stem cell banking services, and the integration of artificial intelligence and 3D bioprinting technologies. The COVID-19 pandemic has further highlighted the importance of iPSCs in infectious disease research. Looking ahead, continued investments in research and development, addressing ethical concerns, enhancing manufacturing processes, fostering regulatory support, and promoting awareness and education will shape the future of the MEA iPSCs market, unlocking new therapeutic possibilities and improving patient outcomes.
What is Induced Pluripotent Stem Cells (iPSCs)?
Induced Pluripotent Stem Cells (iPSCs) are a type of stem cell that can be generated directly from adult cells. They have the ability to differentiate into various cell types, making them valuable for research and potential therapeutic applications in regenerative medicine and disease modeling.
What are the key players in the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market?
Key players in the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market include companies like Thermo Fisher Scientific, Merck KGaA, and Takara Bio, among others. These companies are involved in the development and commercialization of iPSC technologies and related products.
What are the growth factors driving the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market?
The growth of the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market is driven by increasing investments in stem cell research, advancements in regenerative medicine, and the rising prevalence of chronic diseases that require innovative treatment solutions.
What challenges does the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market face?
The Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market faces challenges such as regulatory hurdles, ethical concerns regarding stem cell research, and the high costs associated with iPSC technology development and application.
What opportunities exist in the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market?
Opportunities in the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market include the potential for breakthroughs in personalized medicine, collaborations between research institutions and biotech companies, and the expansion of clinical applications in treating genetic disorders and degenerative diseases.
What trends are shaping the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market?
Trends shaping the Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market include the increasing use of iPSCs in drug discovery and toxicity testing, advancements in gene editing technologies, and a growing focus on ethical sourcing and sustainability in stem cell research.
Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) market
| Segmentation Details | Description |
|---|---|
| Product Type | Research Reagents, Cell Culture Media, Cell Lines, Kits |
| End User | Academic Institutions, Pharmaceutical Companies, Biotechnology Firms, Research Laboratories |
| Application | Drug Discovery, Regenerative Medicine, Disease Modeling, Toxicology Testing |
| Technology | Cell Reprogramming, Gene Editing, Cell Differentiation, Bioprinting |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Middle East and Africa Induced Pluripotent Stem Cells (iPSCs) Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at