444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview:
The LAMEA downstream processing market is a crucial segment within the broader biopharmaceutical industry, encompassing the purification and separation of biotherapeutic products during the manufacturing process. Downstream processing plays a pivotal role in ensuring the production of high-quality and pure biopharmaceuticals, ranging from monoclonal antibodies to vaccines. This market is characterized by the adoption of advanced technologies and the implementation of stringent quality control measures to meet regulatory standards.
Meaning:
Downstream processing in biopharmaceutical manufacturing refers to the steps involved in purifying and isolating the desired product from the complex mixture produced during the upstream fermentation or cell culture process. It involves various techniques such as chromatography, filtration, and centrifugation to separate and purify the target biomolecules, ensuring the final product meets quality and safety requirements.
Executive Summary:
The LAMEA downstream processing market has witnessed significant growth in recent years, driven by the increasing demand for biopharmaceuticals, advancements in purification technologies, and the expansion of the biotechnology industry in the region. As a critical stage in biopharmaceutical production, downstream processing requires continuous innovation and adherence to regulatory guidelines to ensure the safety and efficacy of the final products.
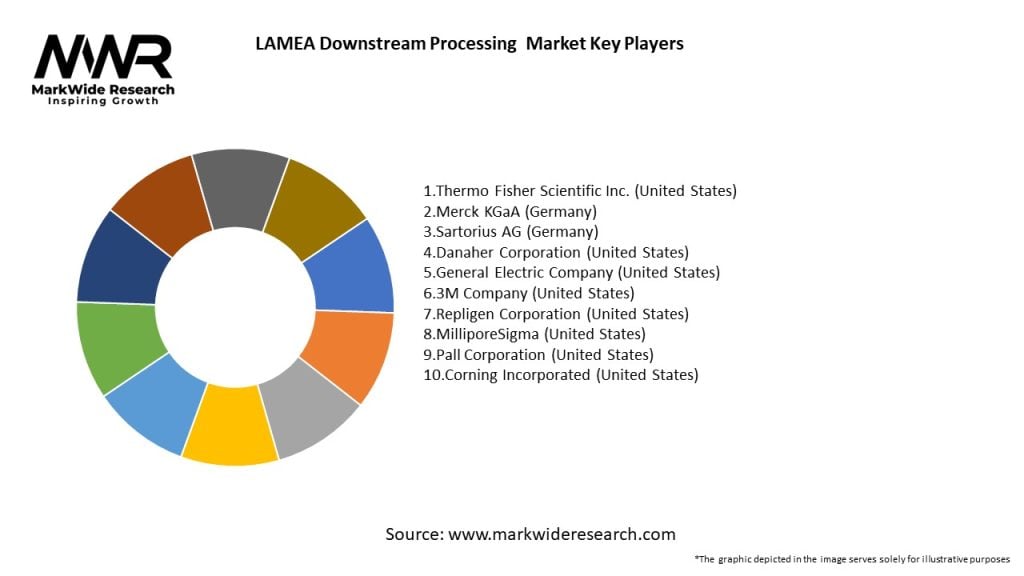
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
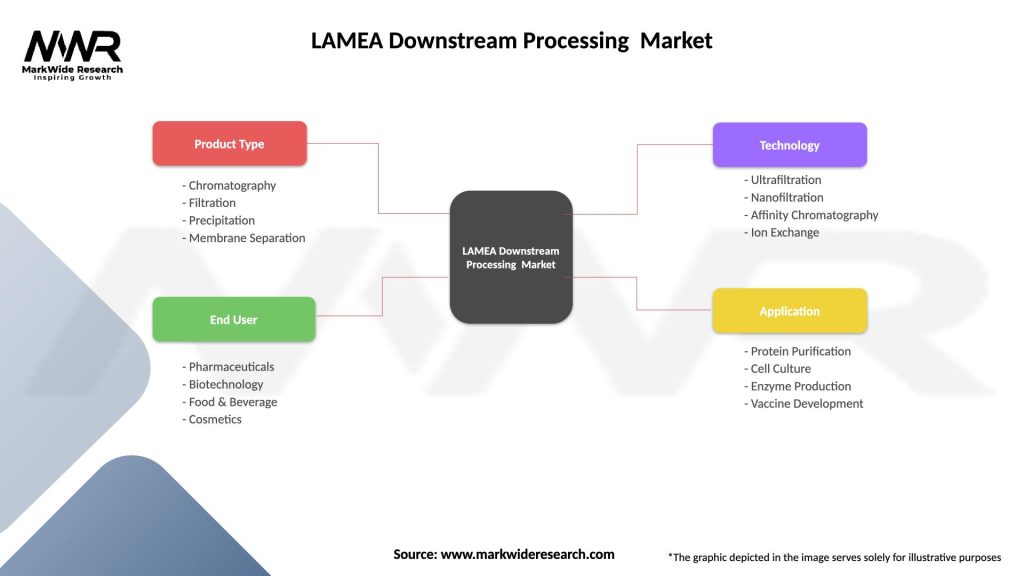
Market Dynamics:
The LAMEA downstream processing market operates in a dynamic environment influenced by factors such as technological advancements, market consolidation, regulatory changes, and industry collaborations. These dynamics shape the market landscape and require industry participants to adapt and innovate to maintain competitiveness.
Regional Analysis:
The downstream processing market in LAMEA exhibits regional variations influenced by economic conditions, healthcare infrastructure, and regulatory frameworks. Key regions contributing to the market include:
Competitive Landscape:
Leading Companies in LAMEA Downstream Processing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation:
The LAMEA downstream processing market can be segmented based on various factors, including:
Segmentation provides a detailed understanding of the market dynamics, allowing companies to tailor their strategies to specific customer needs and industry trends.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders: The LAMEA downstream processing market offers several benefits for industry participants and stakeholders:
SWOT Analysis:
A SWOT analysis provides an overview of the LAMEA downstream processing market’s strengths, weaknesses, opportunities, and threats:
Understanding these factors through a SWOT analysis helps industry participants make informed decisions, capitalize on strengths, address weaknesses, leverage opportunities, and mitigate potential threats.
Market Key Trends:
Covid-19 Impact:
The COVID-19 pandemic has had varied impacts on the LAMEA downstream processing market. While the initial disruption in the supply chain and manufacturing processes was observed, the biopharmaceutical industry demonstrated resilience in adapting to the challenges. Key impacts include:
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The future outlook for the LAMEA downstream processing market is optimistic, with anticipated growth driven by the expanding biopharmaceutical industry, technological innovations, and increasing investments. Continuous advancements in purification technologies, the adoption of single-use solutions, and strategic collaborations are expected to shape the market’s trajectory.
Conclusion:
The LAMEA downstream processing market represents a critical component of the biopharmaceutical industry, ensuring the production of pure and high-quality biotherapeutics. With a focus on technological advancements, regulatory compliance, and strategic collaborations, the market is poised for sustained growth. The industry’s ability to address challenges, capitalize on emerging opportunities, and adapt to evolving market dynamics will be instrumental in shaping its future success.
What is Downstream Processing?
Downstream processing refers to the purification and recovery processes involved in the production of biopharmaceuticals, including the separation of biological products from the fermentation broth. This process is crucial for ensuring the quality and efficacy of products such as vaccines, monoclonal antibodies, and enzymes.
What are the key players in the LAMEA Downstream Processing Market?
Key players in the LAMEA Downstream Processing Market include companies like Merck KGaA, Sartorius AG, and Thermo Fisher Scientific. These companies are known for their innovative technologies and solutions in bioprocessing and purification, among others.
What are the growth factors driving the LAMEA Downstream Processing Market?
The growth of the LAMEA Downstream Processing Market is driven by the increasing demand for biopharmaceuticals, advancements in purification technologies, and the rise in research and development activities in the biotechnology sector. Additionally, the growing prevalence of chronic diseases is fueling the need for effective therapeutic solutions.
What challenges does the LAMEA Downstream Processing Market face?
The LAMEA Downstream Processing Market faces challenges such as high operational costs, regulatory complexities, and the need for skilled personnel. These factors can hinder the efficiency and scalability of downstream processing operations.
What opportunities exist in the LAMEA Downstream Processing Market?
Opportunities in the LAMEA Downstream Processing Market include the expansion of biopharmaceutical manufacturing facilities, the adoption of single-use technologies, and the increasing focus on personalized medicine. These trends are expected to enhance production efficiency and reduce contamination risks.
What trends are shaping the LAMEA Downstream Processing Market?
Trends shaping the LAMEA Downstream Processing Market include the integration of automation and digital technologies in bioprocessing, the shift towards continuous processing methods, and the growing emphasis on sustainability in manufacturing practices. These innovations aim to improve productivity and reduce environmental impact.
LAMEA Downstream Processing Market
| Segmentation Details | Description |
|---|---|
| Product Type | Chromatography, Filtration, Precipitation, Membrane Separation |
| End User | Pharmaceuticals, Biotechnology, Food & Beverage, Cosmetics |
| Technology | Ultrafiltration, Nanofiltration, Affinity Chromatography, Ion Exchange |
| Application | Protein Purification, Cell Culture, Enzyme Production, Vaccine Development |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in LAMEA Downstream Processing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at