444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The pharmaceutical glycerin-based products market in Japan is a pivotal segment within the pharmaceutical industry, driven by the widespread applications of glycerin in various medicinal formulations and healthcare products. Glycerin, also known as glycerol, serves as a versatile ingredient in pharmaceutical formulations due to its properties such as solubility, stability, and moisturizing effects. The market encompasses a wide range of products, including oral solutions, topical preparations, suppositories, and excipients, catering to diverse healthcare needs and therapeutic indications. With Japan’s aging population and increasing focus on healthcare, the demand for pharmaceutical glycerin-based products is expected to witness steady growth in the coming years.
Meaning
Pharmaceutical glycerin-based products refer to medicinal formulations and healthcare products that contain glycerin as a key ingredient. Glycerin, a colorless and odorless liquid derived from natural fats and oils, serves multiple functions in pharmaceutical formulations, including as a solvent, humectant, emollient, and lubricant. Pharmaceutical glycerin-based products encompass a wide range of dosage forms, including oral solutions, topical preparations, suppositories, and excipients, used for various therapeutic purposes such as hydration, lubrication, and drug delivery in the pharmaceutical and healthcare industries.
Executive Summary
The pharmaceutical glycerin-based products market in Japan is poised for growth driven by factors such as the aging population, increasing healthcare expenditures, and growing demand for pharmaceutical formulations with enhanced efficacy and patient compliance. Glycerin-based products offer unique advantages such as biocompatibility, stability, and versatility, making them essential components of many medicinal formulations and healthcare products. Despite challenges such as regulatory requirements, competition, and product differentiation, the market presents significant opportunities for industry participants to innovate, expand their product portfolios, and meet the evolving healthcare needs of the Japanese population.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The pharmaceutical glycerin-based products market in Japan operates in a dynamic and evolving environment influenced by factors such as demographic trends, healthcare reforms, technological advancements, regulatory developments, and competitive dynamics. Understanding these market dynamics is essential for industry participants to identify opportunities, mitigate risks, and develop strategies for sustainable growth and success in the Japanese market.
Regional Analysis
The pharmaceutical glycerin-based products market in Japan exhibits regional variations in demand, adoption, and market dynamics, influenced by factors such as population density, healthcare infrastructure, economic conditions, and regulatory frameworks. Regions with higher concentrations of healthcare facilities, research institutions, and urban populations may present greater opportunities for market penetration, distribution, and sales of pharmaceutical glycerin-based products, requiring tailored strategies, resources, and investments to address regional market needs and preferences.
Competitive Landscape
Leading Companies in Japan Pharmaceutical Glycerin Based Products Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
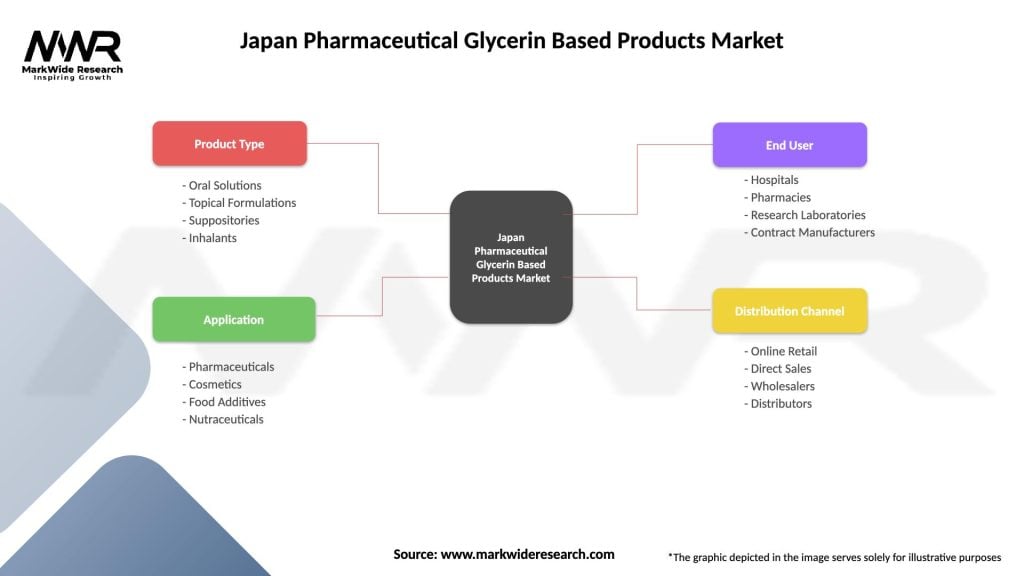
Segmentation
The pharmaceutical glycerin-based products market in Japan can be segmented based on various factors such as:
Segmentation provides a more detailed understanding of market dynamics, consumer preferences, and competitive landscapes, enabling industry participants to tailor their strategies, products, and services to specific market segments and customer needs.
Category-wise Insights
Understanding category-wise insights enables industry participants to identify market opportunities, develop targeted products, and address specific therapeutic needs and patient populations in the Japanese market for pharmaceutical glycerin-based products.
Key Benefits for Industry Participants and Stakeholders
Understanding the key benefits of pharmaceutical glycerin-based products enables industry participants and stakeholders to leverage these advantages to differentiate their products, meet market demands, and achieve sustainable growth and success in the Japanese market.
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Understanding the SWOT analysis helps industry participants and stakeholders identify internal strengths and weaknesses, external opportunities and threats, and develop strategies to capitalize on strengths, mitigate weaknesses, leverage opportunities, and address threats in the Japanese market for pharmaceutical glycerin-based products.
Market Key Trends
Understanding market key trends enables industry participants to anticipate market shifts, consumer preferences, and regulatory developments, and align their strategies, products, and services with emerging opportunities and market demands in the Japanese market for pharmaceutical glycerin-based products.
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the pharmaceutical glycerin-based products market in Japan. While the initial phase of the pandemic led to disruptions in supply chains, manufacturing operations, and distribution channels, the market quickly adapted to the changing healthcare landscape. Some key impacts of COVID-19 on the market include:
Understanding the impact of COVID-19 on the market helps industry participants and stakeholders adapt to new challenges, seize emerging opportunities, and navigate the evolving healthcare landscape in Japan.
Key Industry Developments
Understanding key industry developments helps industry participants and stakeholders anticipate market trends, regulatory changes, and competitive dynamics, and align their strategies, investments, and operations with emerging opportunities and challenges in the Japanese market for pharmaceutical glycerin-based products.
Analyst Suggestions
Implementing these analyst suggestions helps industry participants and stakeholders navigate market challenges, seize growth opportunities, and achieve sustainable success in the Japanese market for pharmaceutical glycerin-based products.
Future Outlook
The pharmaceutical glycerin-based products market in Japan is poised for growth driven by factors such as the aging population, increasing healthcare expenditures, and growing demand for innovative formulations and personalized treatments. However, challenges such as regulatory compliance, market competition, and product differentiation require industry participants to innovate, collaborate, and adapt to changing market dynamics. The future outlook for the market is characterized by:
The future outlook for the pharmaceutical glycerin-based products market in Japan is characterized by growth opportunities, regulatory challenges, technological advancements, and sustainability initiatives that require industry participants to innovate, collaborate, and adapt to changing market dynamics for sustainable success and market leadership.
Conclusion
The pharmaceutical glycerin-based products market in Japan is a dynamic and evolving segment within the healthcare industry, driven by demographic trends, healthcare reforms, technological advancements, and changing consumer preferences. Glycerin-based products offer versatility, efficacy, and safety in various therapeutic areas, catering to diverse healthcare needs and patient populations. Despite challenges such as regulatory compliance, market competition, and product differentiation, the market presents significant opportunities for industry stakeholders to innovate, collaborate, and address unmet medical needs in the Japanese market. By investing in R&D, ensuring regulatory compliance, embracing sustainability, and leveraging digital innovation, industry participants can achieve sustainable growth and success in the Japanese market for pharmaceutical glycerin-based products, contributing to improved patient outcomes and healthcare delivery in Japan and beyond.
What is Pharmaceutical Glycerin Based Products?
Pharmaceutical glycerin based products are formulations that utilize glycerin as a key ingredient, often used for its moisturizing, solvent, and preservative properties in various pharmaceutical applications, including topical ointments and oral solutions.
What are the key players in the Japan Pharmaceutical Glycerin Based Products Market?
Key players in the Japan Pharmaceutical Glycerin Based Products Market include companies like KMG Chemicals, Ashland Global Holdings, and Croda International, which are known for their innovative glycerin formulations and extensive distribution networks, among others.
What are the growth factors driving the Japan Pharmaceutical Glycerin Based Products Market?
The growth of the Japan Pharmaceutical Glycerin Based Products Market is driven by increasing demand for natural and effective excipients in drug formulations, rising consumer awareness regarding skin health, and the expansion of the pharmaceutical industry in Japan.
What challenges does the Japan Pharmaceutical Glycerin Based Products Market face?
Challenges in the Japan Pharmaceutical Glycerin Based Products Market include stringent regulatory requirements for product safety and efficacy, competition from synthetic alternatives, and fluctuations in raw material prices affecting production costs.
What opportunities exist in the Japan Pharmaceutical Glycerin Based Products Market?
Opportunities in the Japan Pharmaceutical Glycerin Based Products Market include the growing trend towards natural and organic products, advancements in glycerin extraction technologies, and increasing applications in the cosmetic and personal care sectors.
What trends are shaping the Japan Pharmaceutical Glycerin Based Products Market?
Trends shaping the Japan Pharmaceutical Glycerin Based Products Market include the rising popularity of multifunctional ingredients, the shift towards sustainable sourcing of glycerin, and innovations in product formulations that enhance bioavailability and efficacy.
Japan Pharmaceutical Glycerin Based Products Market
| Segmentation Details | Description |
|---|---|
| Product Type | Oral Solutions, Topical Formulations, Suppositories, Inhalants |
| Application | Pharmaceuticals, Cosmetics, Food Additives, Nutraceuticals |
| End User | Hospitals, Pharmacies, Research Laboratories, Contract Manufacturers |
| Distribution Channel | Online Retail, Direct Sales, Wholesalers, Distributors |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Japan Pharmaceutical Glycerin Based Products Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at