444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Isothermal Nucleic Acid Amplification Technology (INAAT) is a rapidly evolving diagnostic technique that has gained significant attention in the healthcare industry. It allows for the amplification and detection of nucleic acids at a constant temperature, eliminating the need for complex thermal cycling processes required in traditional polymerase chain reaction (PCR) methods. The INAAT market has witnessed substantial growth in recent years due to its advantages in terms of simplicity, speed, sensitivity, and cost-effectiveness.
Isothermal Nucleic Acid Amplification Technology refers to a group of molecular biology techniques that enable the amplification of specific DNA or RNA sequences without the need for thermal cycling. Unlike PCR, which requires multiple temperature cycles to amplify DNA, isothermal methods operate at a constant temperature, simplifying the amplification process. This technology has revolutionized molecular diagnostics by offering faster and more accessible testing solutions.
Executive Summary
The Isothermal Nucleic Acid Amplification Technology market has experienced remarkable growth over the past few years, driven by the increasing demand for rapid and accurate molecular diagnostic tests. The technology’s ability to amplify nucleic acids at a constant temperature has made it an attractive alternative to PCR, particularly in resource-limited settings. With ongoing advancements and innovations, the INAAT market is poised for further expansion in the coming years.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The INAAT market is dynamic, driven by advancements in technology, evolving regulatory frameworks, and changing healthcare landscapes. The market is witnessing increased competition, with several companies investing in research and development to enhance the performance and versatility of INAAT-based tests. Moreover, strategic partnerships and collaborations are shaping the market dynamics, leading to the development of innovative solutions and expanded market reach.
Regional Analysis
The INAAT market exhibits regional variations, influenced by factors such as healthcare infrastructure, disease prevalence, and regulatory environment. North America and Europe have been early adopters of INAAT technology, driven by robust healthcare systems and high awareness levels. Asia Pacific is expected to witness significant growth due to the increasing burden of infectious diseases and the rising adoption of molecular diagnostics in the region.
Competitive Landscape
Leading Companies in the Isothermal Nucleic Acid Amplification Technology Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
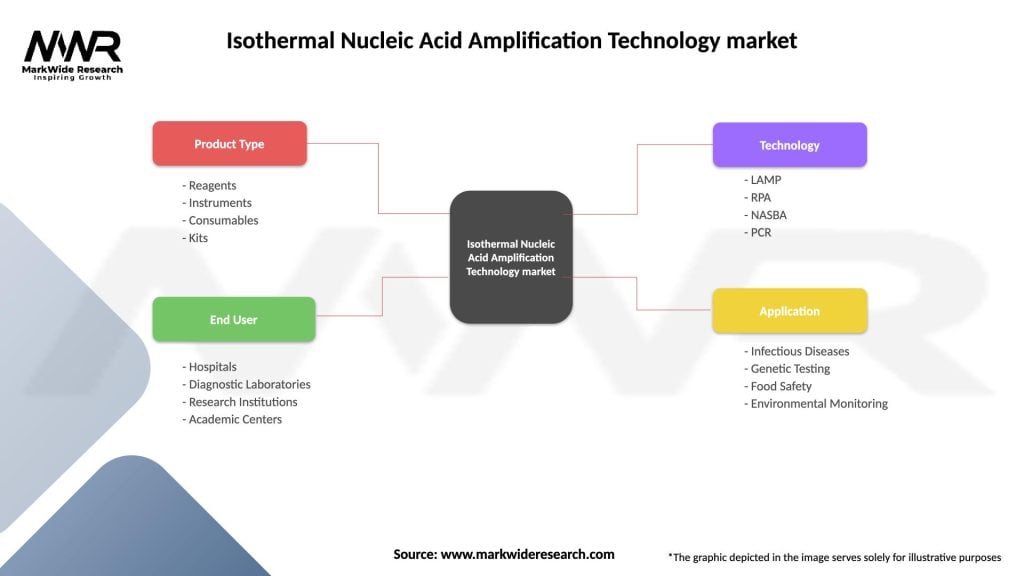
The INAAT market can be segmented based on technology, application, end-user, and region. By technology, the market can be divided into loop-mediated isothermal amplification (LAMP), nucleic acid sequence-based amplification (NASBA), and others. Applications of INAAT include infectious disease diagnostics, genetic testing, oncology, and others. End-users of INAAT technology include hospitals and clinics, research institutes, diagnostic laboratories, and others.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has accelerated the adoption of INAAT technology for the detection of SARS-CoV-2, the virus responsible for the disease. The simplicity, speed, and accuracy of INAAT-based tests have made them valuable tools in mass testing efforts, especially in settings where PCR testing capacity is limited. The pandemic has highlighted the importance of rapid and accessible diagnostic solutions, driving further research and development in the INAAT market.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the INAAT market looks promising, with continuous advancements and innovations expected in the field. The adoption of INAAT technology is likely to increase, driven by the need for rapid, accurate, and decentralized diagnostic solutions. Ongoing research and development efforts aim to further enhance the performance, multiplexing capabilities, and compatibility of INAAT-based tests, paving the way for their wider integration into routine diagnostic practices.
Conclusion
Isothermal Nucleic Acid Amplification Technology has revolutionized the field of molecular diagnostics by providing faster, simpler, and cost-effective solutions for nucleic acid amplification and detection. The market has experienced significant growth, driven by the increasing demand for rapid and accurate diagnostic tests. As awareness and adoption continue to expand, the INAAT market is expected to witness further advancements, collaborations, and regulatory approvals, contributing to improved healthcare outcomes worldwide.
What is Isothermal Nucleic Acid Amplification Technology?
Isothermal Nucleic Acid Amplification Technology refers to a method used to amplify nucleic acids at a constant temperature, enabling rapid and efficient detection of pathogens and genetic material. This technology is widely utilized in diagnostics, research, and biotechnology applications.
What are the key players in the Isothermal Nucleic Acid Amplification Technology market?
Key players in the Isothermal Nucleic Acid Amplification Technology market include companies like Abbott Laboratories, Thermo Fisher Scientific, and QIAGEN, which are known for their innovative diagnostic solutions and nucleic acid testing products, among others.
What are the growth factors driving the Isothermal Nucleic Acid Amplification Technology market?
The growth of the Isothermal Nucleic Acid Amplification Technology market is driven by the increasing demand for rapid diagnostic tests, advancements in molecular biology techniques, and the rising prevalence of infectious diseases. These factors contribute to the technology’s adoption in clinical and research settings.
What challenges does the Isothermal Nucleic Acid Amplification Technology market face?
Challenges in the Isothermal Nucleic Acid Amplification Technology market include the need for standardization of protocols, potential for contamination during testing, and the requirement for skilled personnel to interpret results. These issues can hinder widespread adoption in some regions.
What opportunities exist in the Isothermal Nucleic Acid Amplification Technology market?
Opportunities in the Isothermal Nucleic Acid Amplification Technology market include the development of point-of-care testing devices, integration with digital health technologies, and expansion into emerging markets. These trends can enhance accessibility and efficiency in diagnostics.
What trends are shaping the Isothermal Nucleic Acid Amplification Technology market?
Trends shaping the Isothermal Nucleic Acid Amplification Technology market include the increasing use of microfluidics for sample processing, the rise of multiplexing capabilities for simultaneous detection of multiple targets, and the growing interest in personalized medicine. These innovations are enhancing the technology’s applications.
Isothermal Nucleic Acid Amplification Technology market
| Segmentation Details | Description |
|---|---|
| Product Type | Reagents, Instruments, Consumables, Kits |
| End User | Hospitals, Diagnostic Laboratories, Research Institutions, Academic Centers |
| Technology | LAMP, RPA, NASBA, PCR |
| Application | Infectious Diseases, Genetic Testing, Food Safety, Environmental Monitoring |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Isothermal Nucleic Acid Amplification Technology Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at