444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2150
The India cardiac surgery instruments market represents a rapidly expanding segment within the country’s medical device industry, driven by increasing cardiovascular disease prevalence and advancing surgical technologies. India’s healthcare infrastructure has witnessed remarkable transformation, with cardiac surgery instruments becoming increasingly sophisticated and specialized to meet growing demand from hospitals and cardiac centers nationwide.
Market dynamics indicate substantial growth potential, with the sector experiencing a robust 8.2% CAGR as healthcare facilities modernize their cardiac surgical capabilities. The market encompasses a comprehensive range of instruments including cardiac catheters, stents, pacemakers, defibrillators, and specialized surgical tools designed for various cardiac procedures.
Healthcare expenditure in India has increased significantly, with approximately 62% of cardiac surgery centers upgrading their instrument portfolios to incorporate advanced technologies. This transformation reflects the country’s commitment to improving cardiovascular care outcomes and reducing mortality rates associated with heart diseases.
Regional distribution shows concentrated growth in metropolitan areas, with 45% of market activity centered in major cities like Mumbai, Delhi, Chennai, and Bangalore. These urban centers serve as hubs for advanced cardiac care, attracting patients from across the country and driving demand for sophisticated surgical instruments.
The India cardiac surgery instruments market refers to the comprehensive ecosystem of medical devices, tools, and equipment specifically designed for cardiovascular surgical procedures within the Indian healthcare system. This market encompasses both invasive and minimally invasive cardiac surgery instruments used in hospitals, specialty cardiac centers, and ambulatory surgical facilities across the country.
Cardiac surgery instruments include a diverse range of products from basic surgical tools like forceps and scissors to highly sophisticated devices such as heart-lung machines, intra-aortic balloon pumps, and robotic surgical systems. These instruments enable cardiac surgeons to perform complex procedures including coronary artery bypass grafting, valve replacements, and congenital heart defect corrections.
Market scope extends beyond traditional surgical instruments to include diagnostic equipment, monitoring devices, and post-operative care tools that support comprehensive cardiac treatment protocols. The integration of digital technologies and artificial intelligence has further expanded the definition to include smart instruments capable of real-time data collection and analysis.
India’s cardiac surgery instruments market demonstrates exceptional growth momentum, positioning itself as a critical component of the nation’s expanding healthcare infrastructure. The market benefits from increasing awareness of cardiovascular health, government initiatives promoting healthcare accessibility, and rising disposable incomes enabling better medical care access.
Key growth drivers include the alarming rise in cardiovascular diseases, with heart conditions affecting approximately 54 million Indians according to recent health surveys. This demographic shift, combined with lifestyle changes and urbanization, has created unprecedented demand for advanced cardiac surgical interventions and corresponding instrumentation.
Technological advancement represents another crucial factor, with 38% of cardiac centers adopting minimally invasive surgical techniques that require specialized instruments. These procedures offer reduced recovery times, lower infection risks, and improved patient outcomes, driving hospitals to invest in cutting-edge surgical equipment.
Market segmentation reveals diverse opportunities across product categories, with interventional cardiology devices showing particularly strong growth. The sector benefits from both domestic manufacturing initiatives and strategic partnerships with international medical device companies, creating a robust supply chain ecosystem.
Strategic market insights reveal several transformative trends shaping India’s cardiac surgery instruments landscape. The integration of digital health technologies with traditional surgical instruments has created new opportunities for enhanced patient monitoring and surgical precision.
Cardiovascular disease prevalence stands as the primary driver propelling India’s cardiac surgery instruments market forward. The country faces a significant burden of heart diseases, with changing dietary patterns, sedentary lifestyles, and increased stress levels contributing to rising incidence rates across all age groups.
Government healthcare initiatives have substantially boosted market growth through schemes like Ayushman Bharat and National Health Mission. These programs have improved healthcare accessibility for millions of Indians, directly translating to increased demand for cardiac surgical procedures and associated instruments.
Aging population demographics represent another crucial driver, with India’s elderly population expected to grow significantly over the coming decades. This demographic shift correlates directly with increased cardiovascular disease prevalence, necessitating expanded cardiac surgery capabilities and instrument requirements.
Healthcare infrastructure expansion continues accelerating across urban and rural areas, with new hospitals and cardiac centers requiring comprehensive instrument inventories. This expansion includes both private healthcare facilities and government-supported medical institutions, creating diverse market opportunities.
Medical education advancement has produced more qualified cardiac surgeons and healthcare professionals, increasing the capacity for complex cardiac procedures. This skilled workforce expansion directly drives demand for sophisticated surgical instruments and training equipment.
Insurance coverage expansion has made cardiac procedures more accessible to middle-class populations, removing financial barriers that previously limited treatment options. Improved insurance penetration has democratized access to advanced cardiac care and corresponding surgical instruments.
High instrument costs present significant challenges for many healthcare facilities, particularly smaller hospitals and clinics with limited budgets. Advanced cardiac surgery instruments often require substantial capital investments, creating barriers to market penetration in cost-sensitive segments.
Skilled workforce shortages limit the effective utilization of sophisticated cardiac instruments, as complex equipment requires specialized training and expertise. Many healthcare facilities struggle to recruit and retain qualified technicians and surgeons capable of operating advanced cardiac surgical equipment.
Regulatory complexities can delay product approvals and market entry for new cardiac instruments. India’s evolving medical device regulations, while necessary for patient safety, sometimes create lengthy approval processes that impact market dynamics and innovation timelines.
Infrastructure limitations in rural and semi-urban areas restrict market expansion opportunities. Many regions lack adequate power supply, maintenance facilities, and technical support networks necessary for sophisticated cardiac instruments to function effectively.
Import dependencies for critical components and advanced technologies create supply chain vulnerabilities and cost pressures. Currency fluctuations and international trade dynamics can significantly impact instrument pricing and availability in the Indian market.
Maintenance and service challenges pose ongoing concerns for healthcare facilities investing in complex cardiac instruments. Limited local service networks and high maintenance costs can impact the total cost of ownership and operational efficiency.
Digital health integration presents unprecedented opportunities for cardiac instrument manufacturers to develop smart, connected devices that enhance surgical outcomes and patient monitoring. The convergence of artificial intelligence, machine learning, and traditional surgical instruments opens new avenues for innovation and market differentiation.
Rural healthcare expansion offers significant untapped potential, with government initiatives focusing on improving cardiac care accessibility in underserved regions. This expansion creates opportunities for cost-effective, portable cardiac instruments designed for resource-constrained environments.
Medical tourism growth positions India as a global destination for cardiac procedures, driving demand for world-class surgical instruments that meet international standards. This trend creates opportunities for premium instrument segments and specialized surgical equipment.
Telemedicine integration enables remote monitoring and consultation capabilities, creating demand for cardiac instruments with telecommunication features. This integration supports both urban and rural healthcare delivery models, expanding market reach and accessibility.
Public-private partnerships offer collaborative opportunities for instrument manufacturers to participate in large-scale healthcare infrastructure projects. These partnerships can accelerate market penetration while supporting national healthcare objectives.
Research and development initiatives supported by government and private funding create opportunities for indigenous innovation in cardiac instrument design and manufacturing. This focus on local R&D can reduce import dependencies while fostering technological advancement.
Supply chain evolution continues reshaping the cardiac surgery instruments market, with manufacturers developing more resilient and efficient distribution networks. The COVID-19 pandemic highlighted the importance of supply chain diversification and local manufacturing capabilities, leading to strategic shifts in sourcing and production strategies.
Technology convergence drives market dynamics through the integration of multiple technologies within single instrument platforms. Modern cardiac instruments increasingly combine diagnostic, therapeutic, and monitoring capabilities, creating more comprehensive solutions for healthcare providers.
Competitive landscape shifts reflect changing market dynamics, with both international and domestic players adapting strategies to capture emerging opportunities. The market witnesses increased collaboration between global technology leaders and local manufacturing partners, creating hybrid business models.
Regulatory environment evolution influences market dynamics through updated standards and compliance requirements. Recent regulatory changes emphasize patient safety, quality assurance, and post-market surveillance, impacting product development and commercialization strategies.
Healthcare delivery model transformation affects instrument requirements and market dynamics. The shift toward value-based care, outcome-focused treatments, and patient-centric approaches drives demand for instruments that demonstrate clear clinical and economic benefits.
Investment patterns show increased focus on cardiac healthcare infrastructure, with both domestic and international investors recognizing the sector’s growth potential. This capital influx supports market expansion while enabling technology adoption and facility modernization.
Comprehensive market analysis employs multiple research methodologies to ensure accurate and reliable insights into India’s cardiac surgery instruments market. The research approach combines primary and secondary data collection methods, providing a holistic view of market dynamics, trends, and growth opportunities.
Primary research activities include extensive interviews with key stakeholders across the cardiac surgery instruments value chain. These stakeholders encompass cardiac surgeons, hospital administrators, medical device distributors, regulatory officials, and industry executives who provide firsthand insights into market conditions and future prospects.
Secondary research methodology involves systematic analysis of published reports, government databases, medical journals, industry publications, and regulatory documents. This approach ensures comprehensive coverage of market segments, competitive landscape, and regulatory environment factors influencing market development.
Data validation processes employ triangulation techniques to verify information accuracy and reliability. Multiple data sources are cross-referenced to ensure consistency and eliminate potential biases or inaccuracies in market assessments and projections.
Quantitative analysis techniques include statistical modeling, trend analysis, and market sizing methodologies that provide numerical insights into market performance and growth trajectories. These techniques help identify patterns and correlations within market data.
Qualitative research components focus on understanding market dynamics, stakeholder perspectives, and emerging trends that may not be captured through quantitative methods alone. This approach provides context and depth to numerical findings.
Northern India represents a significant portion of the cardiac surgery instruments market, with Delhi NCR serving as a major healthcare hub. The region benefits from established medical infrastructure, government healthcare initiatives, and proximity to regulatory authorities, creating favorable conditions for market growth.
Western India demonstrates strong market presence, particularly in Maharashtra and Gujarat, where 35% of cardiac procedures are performed in major metropolitan centers. Mumbai’s status as a financial capital attracts significant healthcare investments, while Pune emerges as a medical device manufacturing hub.
Southern India leads in cardiac surgery innovation and medical tourism, with cities like Chennai, Bangalore, and Hyderabad hosting world-class cardiac centers. The region accounts for approximately 28% of cardiac instrument demand, driven by advanced healthcare infrastructure and skilled medical professionals.
Eastern India shows growing market potential, with Kolkata serving as the regional healthcare center. Government initiatives to improve healthcare accessibility in eastern states create opportunities for cardiac instrument market expansion, though infrastructure challenges remain.
Central India presents emerging opportunities as healthcare infrastructure develops in cities like Bhopal, Indore, and Nagpur. The region benefits from government focus on improving healthcare accessibility in underserved areas, creating demand for cost-effective cardiac instruments.
Tier-2 and Tier-3 cities represent significant growth opportunities, with 42% of new cardiac centers being established outside major metropolitan areas. This expansion reflects government initiatives to decentralize healthcare delivery and improve accessibility for rural populations.
Market leadership in India’s cardiac surgery instruments sector reflects a diverse ecosystem of international and domestic players, each contributing unique strengths and capabilities. The competitive landscape continues evolving as companies adapt strategies to capture emerging opportunities and address changing market dynamics.
Strategic partnerships between international companies and local manufacturers have become increasingly common, enabling technology transfer while leveraging local market knowledge and cost advantages. These collaborations support market expansion while addressing regulatory and distribution challenges.
Innovation focus drives competitive differentiation, with companies investing heavily in research and development to create next-generation cardiac instruments. This innovation emphasis includes digital health integration, artificial intelligence applications, and improved patient outcomes.
Product-based segmentation reveals diverse market opportunities across multiple cardiac instrument categories, each serving specific surgical procedures and clinical requirements. This segmentation approach helps identify growth areas and market dynamics within different product segments.
By Product Type:
By End User:
By Procedure Type:
Interventional cardiology devices dominate the market landscape, driven by the high prevalence of coronary artery disease and increasing adoption of minimally invasive procedures. This category benefits from technological advancement in drug-eluting stents, bioresorbable scaffolds, and advanced catheter technologies.
Cardiac surgery instruments show steady growth supported by complex surgical procedures and technological innovation. The segment includes traditional surgical tools alongside advanced robotic surgical systems and computer-assisted surgical instruments that enhance precision and outcomes.
Cardiac rhythm management devices experience robust growth due to increasing awareness of arrhythmia treatment options and aging population demographics. This category includes both implantable devices and external monitoring systems that support comprehensive cardiac rhythm management.
Monitoring and diagnostic equipment represents a rapidly expanding category, driven by emphasis on preventive care and early intervention strategies. Advanced monitoring systems with artificial intelligence capabilities and remote monitoring features show particular growth potential.
Cardiac assist devices constitute a specialized but growing segment, particularly in tertiary care hospitals performing complex cardiac procedures. These devices support patients during high-risk procedures and recovery periods, requiring specialized training and infrastructure.
Pediatric cardiac instruments represent a niche but important category, addressing the unique requirements of congenital heart disease treatment. This segment requires specialized sizing, materials, and design considerations for pediatric patients.
Healthcare providers benefit significantly from advanced cardiac surgery instruments through improved patient outcomes, reduced procedure times, and enhanced surgical precision. Modern instruments enable minimally invasive approaches that reduce patient trauma, accelerate recovery, and improve overall treatment success rates.
Patients experience substantial advantages including reduced surgical risks, shorter hospital stays, and faster recovery times. Advanced cardiac instruments enable procedures that were previously impossible or extremely high-risk, expanding treatment options for complex cardiac conditions.
Surgeons and medical professionals gain access to sophisticated tools that enhance their capabilities and improve procedural outcomes. Training programs and educational initiatives associated with new instruments help advance medical expertise and surgical techniques.
Healthcare institutions achieve operational benefits through improved efficiency, reduced complication rates, and enhanced reputation for quality care. Investment in advanced cardiac instruments often leads to increased patient volumes and improved financial performance.
Medical device manufacturers benefit from growing market opportunities, technological innovation drivers, and potential for strategic partnerships. The expanding market creates opportunities for both established companies and emerging players to develop innovative solutions.
Government and regulatory bodies achieve public health objectives through improved cardiac care accessibility and outcomes. The growth of the cardiac instruments market supports broader healthcare infrastructure development and medical tourism initiatives.
Research institutions benefit from collaboration opportunities with industry partners, access to advanced technologies for clinical research, and potential for developing indigenous innovations that address local market needs.
Strengths:
Weaknesses:
Opportunities:
Threats:
Minimally invasive surgery adoption continues accelerating across India’s cardiac surgery landscape, with 67% of interventional procedures now utilizing minimally invasive techniques. This trend drives demand for specialized instruments designed for catheter-based procedures, robotic surgery systems, and advanced imaging integration.
Digital health integration represents a transformative trend, with cardiac instruments increasingly incorporating connectivity features, data analytics capabilities, and artificial intelligence applications. These smart instruments enable real-time monitoring, predictive analytics, and improved decision-making during surgical procedures.
Personalized medicine approaches influence instrument design and functionality, with growing emphasis on patient-specific solutions and customizable treatment options. This trend includes 3D-printed cardiac devices, patient-specific surgical planning tools, and instruments designed for individual anatomical variations.
Telemedicine integration expands the reach of cardiac care through remote monitoring capabilities and teleconsultation support. Cardiac instruments with telecommunication features enable specialists to provide guidance and monitoring for patients in remote locations.
Sustainability focus drives development of eco-friendly cardiac instruments and packaging solutions. Manufacturers increasingly consider environmental impact in product design, materials selection, and manufacturing processes.
Training and simulation technologies become integral to cardiac instrument adoption, with virtual reality and augmented reality systems supporting surgeon education and procedure planning. These technologies improve surgical outcomes while reducing learning curves for new instruments.
Value-based healthcare models influence instrument selection and procurement decisions, with healthcare providers focusing on instruments that demonstrate clear clinical and economic benefits. This trend emphasizes outcome measurement and cost-effectiveness analysis.
Regulatory framework evolution has significantly impacted the cardiac surgery instruments market, with updated medical device regulations emphasizing patient safety, quality assurance, and post-market surveillance. Recent regulatory changes require enhanced clinical evidence and risk management protocols for cardiac instruments.
Manufacturing localization initiatives have gained momentum through government support and industry investment. Several international companies have established manufacturing facilities in India, reducing import dependency while creating local employment and technology transfer opportunities.
Strategic partnerships between global medical device companies and Indian healthcare providers have accelerated technology adoption and market expansion. These collaborations often include training programs, clinical support, and customized solutions for local market requirements.
Innovation centers and research facilities have been established by major cardiac instrument manufacturers, focusing on developing solutions specifically for Indian market needs. These centers emphasize cost-effective innovations and technologies suitable for diverse healthcare settings.
Digital health initiatives have transformed cardiac instrument capabilities through integration with electronic health records, telemedicine platforms, and mobile health applications. These developments support comprehensive patient care and improved clinical workflows.
Quality certification programs have been implemented to ensure cardiac instruments meet international standards while addressing local regulatory requirements. These programs support market access and build confidence among healthcare providers and patients.
Educational partnerships between instrument manufacturers and medical institutions have enhanced training opportunities for cardiac surgeons and healthcare professionals. These initiatives improve instrument utilization and clinical outcomes while supporting market growth.
MarkWide Research analysis suggests that stakeholders should prioritize investment in digital health integration and artificial intelligence capabilities to remain competitive in the evolving cardiac surgery instruments market. The convergence of traditional surgical instruments with smart technologies represents the most significant opportunity for market differentiation and growth.
Market entry strategies should focus on tier-2 and tier-3 cities where healthcare infrastructure development creates substantial opportunities for cardiac instrument adoption. Companies should develop cost-effective solutions tailored to resource-constrained environments while maintaining quality standards.
Partnership approaches with local healthcare providers and government initiatives can accelerate market penetration while supporting broader healthcare objectives. These collaborations should emphasize training, clinical support, and outcome measurement to demonstrate value proposition.
Technology development should prioritize user-friendly interfaces, reduced training requirements, and improved reliability to address common adoption barriers in the Indian market. Instruments designed for diverse skill levels and infrastructure conditions will achieve broader market acceptance.
Regulatory compliance strategies must anticipate evolving requirements and maintain proactive approach to quality assurance and post-market surveillance. Early engagement with regulatory authorities can streamline approval processes and reduce time-to-market.
Supply chain optimization should focus on reducing import dependencies through local sourcing and manufacturing partnerships. Diversified supply chains will provide resilience against disruptions while supporting cost competitiveness.
Customer education programs should emphasize clinical outcomes, cost-effectiveness, and operational benefits to support adoption decisions. Evidence-based marketing approaches will resonate with healthcare providers focused on value-based care models.
Long-term growth prospects for India’s cardiac surgery instruments market remain exceptionally positive, driven by demographic trends, healthcare infrastructure expansion, and technological advancement. The market is positioned to benefit from sustained government support, increasing healthcare awareness, and rising disposable incomes across the population.
Technology evolution will continue transforming the cardiac instruments landscape, with artificial intelligence, machine learning, and robotics playing increasingly important roles. MWR projects that 75% of cardiac procedures will incorporate some form of digital technology integration within the next decade, creating substantial opportunities for innovative instrument manufacturers.
Market expansion into underserved regions will accelerate as healthcare infrastructure development reaches rural and semi-urban areas. Government initiatives focusing on healthcare accessibility will create new market segments and drive demand for appropriate technology solutions.
International competitiveness of Indian cardiac surgery centers will continue improving, supporting medical tourism growth and driving demand for world-class surgical instruments. This trend will elevate quality standards while creating opportunities for premium instrument segments.
Innovation ecosystem development will support indigenous research and development capabilities, potentially reducing import dependencies while creating export opportunities. Local innovation centers and research partnerships will contribute to technology advancement and market growth.
Regulatory maturation will provide clearer guidelines and streamlined processes for cardiac instrument approval and commercialization. This evolution will support market stability while ensuring patient safety and quality standards.
Sustainability considerations will increasingly influence instrument design, manufacturing, and disposal practices. Environmental consciousness will drive development of eco-friendly solutions and circular economy approaches in the cardiac instruments market.
India’s cardiac surgery instruments market represents a dynamic and rapidly evolving sector with exceptional growth potential driven by demographic trends, technological advancement, and healthcare infrastructure expansion. The market benefits from strong government support, increasing healthcare awareness, and rising cardiovascular disease prevalence that creates sustained demand for advanced surgical instruments.
Market transformation continues through digital health integration, minimally invasive surgery adoption, and personalized medicine approaches that reshape instrument requirements and capabilities. These trends create opportunities for innovative companies while challenging traditional market approaches and business models.
Strategic success in this market requires understanding of local healthcare dynamics, regulatory environment, and diverse customer needs across urban and rural settings. Companies that can balance technological sophistication with cost-effectiveness and accessibility will achieve sustainable competitive advantages.
Future prospects remain highly positive, with the market positioned to benefit from continued healthcare infrastructure development, medical tourism growth, and technological innovation. The convergence of traditional surgical instruments with digital technologies will create new categories and market opportunities while improving patient outcomes and healthcare delivery efficiency.
What is Cardiac Surgery Instruments?
Cardiac Surgery Instruments are specialized tools used in various surgical procedures related to the heart. These instruments include scalpels, forceps, clamps, and suturing devices designed to facilitate complex cardiac surgeries.
What are the key players in the India Cardiac Surgery Instruments Market?
Key players in the India Cardiac Surgery Instruments Market include Medtronic, Boston Scientific, and Abbott Laboratories, among others. These companies are known for their innovative products and significant contributions to cardiac surgery.
What are the growth factors driving the India Cardiac Surgery Instruments Market?
The growth of the India Cardiac Surgery Instruments Market is driven by the increasing prevalence of cardiovascular diseases, advancements in surgical techniques, and the rising demand for minimally invasive surgeries. Additionally, the growing geriatric population contributes to market expansion.
What challenges does the India Cardiac Surgery Instruments Market face?
The India Cardiac Surgery Instruments Market faces challenges such as high costs of advanced surgical instruments and stringent regulatory requirements. Additionally, the lack of skilled professionals in certain regions can hinder market growth.
What opportunities exist in the India Cardiac Surgery Instruments Market?
Opportunities in the India Cardiac Surgery Instruments Market include the development of innovative and cost-effective surgical tools, increasing investments in healthcare infrastructure, and the potential for growth in telemedicine and remote surgeries. These factors can enhance access to cardiac care.
What trends are shaping the India Cardiac Surgery Instruments Market?
Trends in the India Cardiac Surgery Instruments Market include the rise of robotic-assisted surgeries, the integration of digital technologies in surgical instruments, and a focus on patient safety and outcomes. These trends are transforming how cardiac surgeries are performed.
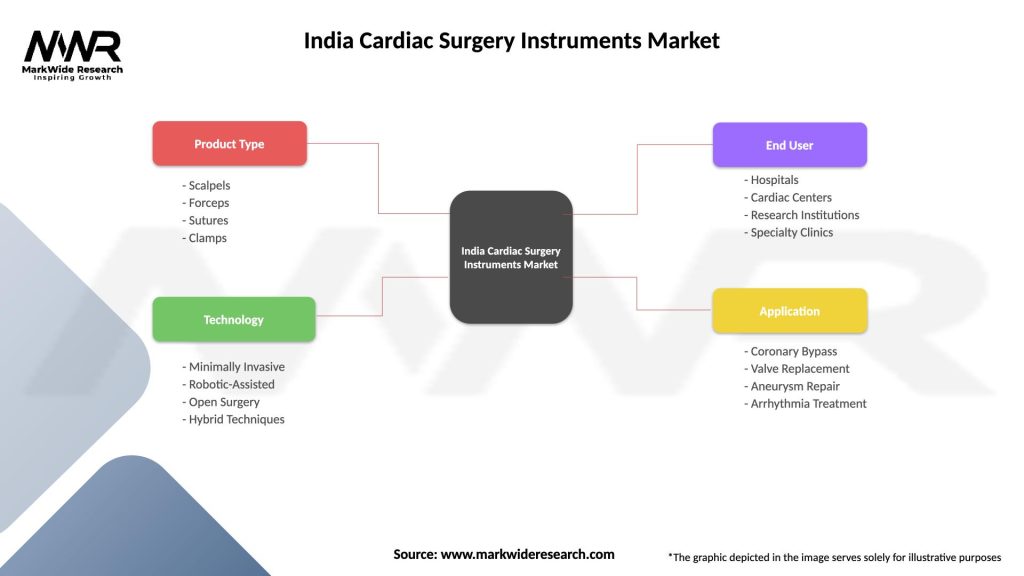
India Cardiac Surgery Instruments Market
| Segmentation Details | Description |
|---|---|
| Product Type | Scalpels, Forceps, Sutures, Clamps |
| Technology | Minimally Invasive, Robotic-Assisted, Open Surgery, Hybrid Techniques |
| End User | Hospitals, Cardiac Centers, Research Institutions, Specialty Clinics |
| Application | Coronary Bypass, Valve Replacement, Aneurysm Repair, Arrhythmia Treatment |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the India Cardiac Surgery Instruments Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at