444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The In-vitro Toxicology Assays market refers to the use of laboratory techniques to assess the toxicity of substances on living cells or tissues outside of the living organism. This field has gained significant importance in recent years due to its ability to provide valuable insights into the potential risks associated with chemical compounds, pharmaceuticals, and consumer products. In-vitro toxicology assays offer a cost-effective and efficient alternative to traditional animal testing methods, while also reducing ethical concerns and regulatory barriers.
Meaning
In-vitro toxicology assays involve the use of specialized tests and techniques to evaluate the potential toxicity of substances on biological systems. These assays are typically performed using cultured cells, tissues, or organoids, which are exposed to various concentrations of a test compound. The responses of the cells or tissues are then measured, allowing researchers to assess the safety and potential adverse effects of the substance being tested.
Executive Summary
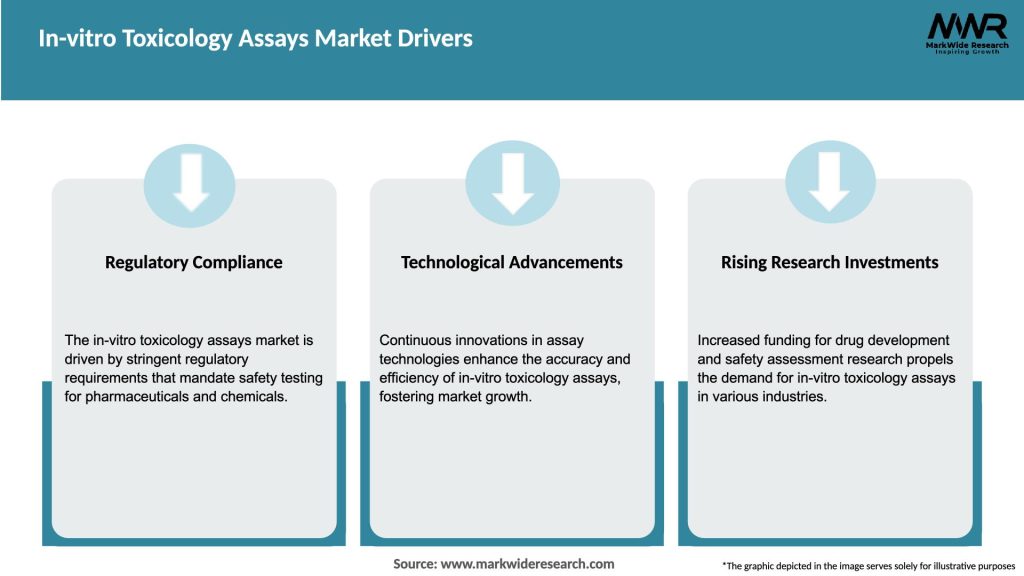
The In-vitro Toxicology Assays market is experiencing substantial growth due to increasing awareness about the importance of safety assessments in various industries, such as pharmaceuticals, cosmetics, and chemicals. The market is driven by factors such as the need for reliable toxicity testing methods, ethical concerns associated with animal testing, and stringent regulatory guidelines. Additionally, advancements in technology, such as high-throughput screening and the development of organ-on-a-chip models, are further propelling market growth.
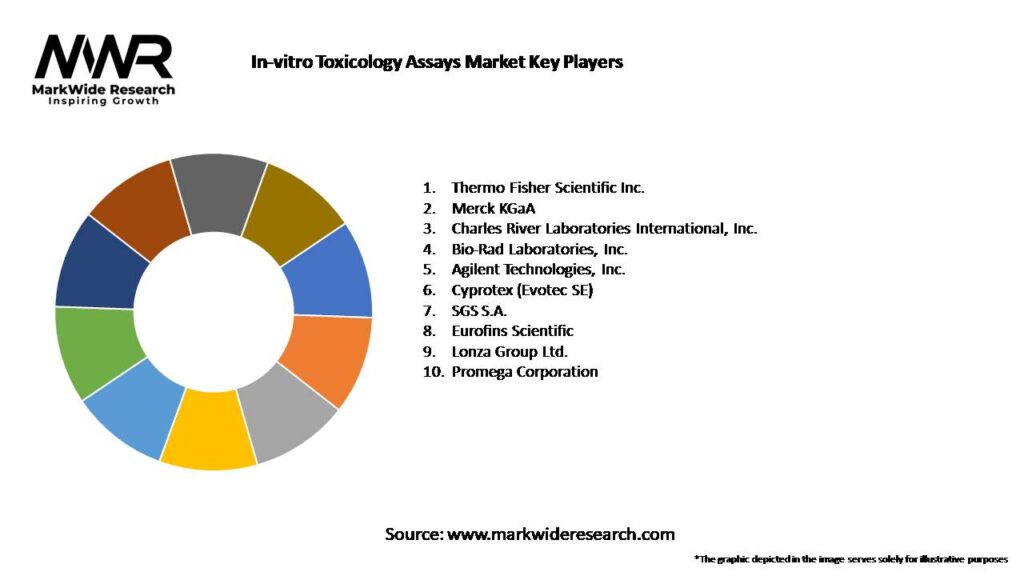
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The In-vitro Toxicology Assays market is highly dynamic, driven by various factors such as regulatory requirements, technological advancements, and market competition. The market is characterized by constant innovation, as industry players strive to develop novel assay systems, improve the predictability of results, and enhance the relevance of in-vitro models. Moreover, strategic collaborations, mergers, and acquisitions are common in this market, allowing companies to expand their product portfolios and strengthen their market presence.
Regional Analysis
Competitive Landscape
Leading Companies in the In-vitro Toxicology Assays Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
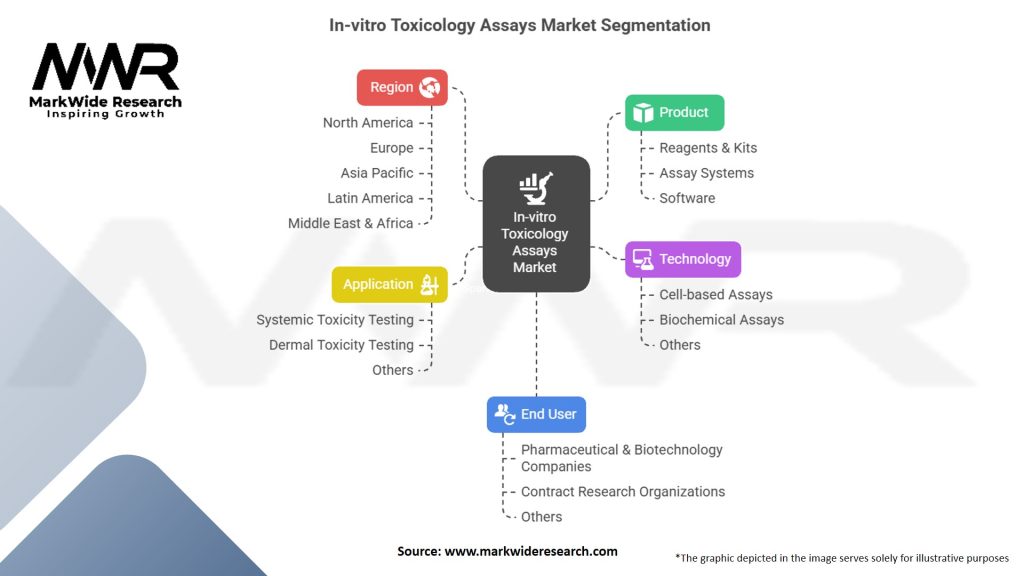
Segmentation
The In-vitro Toxicology Assays market can be segmented based on the following:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a mixed impact on the in-vitro toxicology assays market. While the pandemic disrupted various industries, including pharmaceuticals and research activities, it also highlighted the importance of safety assessments and the need for reliable toxicity testing methods. The demand for in-vitro assays increased, particularly in the development of antiviral drugs and vaccines, as well as in studying the potential adverse effects of Covid-19 therapeutics. However, the pandemic also led to challenges in the supply chain and delays in research activities, affecting the market growth to some extent.
Key Industry Developments
Analyst Suggestions
Future Outlook
The In-vitro Toxicology Assays market is poised for significant growth in the coming years. The increasing emphasis on safety assessments, the need to reduce animal testing, and the implementation of stringent regulatory guidelines will continue to drive the adoption of in-vitro toxicology assays. Technological advancements, such as the integration of AI, organ-on-a-chip models, and 3D cell culture systems, will further enhance the relevance and predictive power of these assays. With expanding applications in pharmaceuticals, cosmetics, and chemicals, the market is expected to witness continued innovation, collaborations, and a competitive landscape.
Conclusion
The In-vitro Toxicology Assays market is experiencing robust growth, driven by the need for reliable toxicity testing methods, ethical concerns related to animal testing, and stringent regulatory requirements. The market offers significant opportunities for industry participants, including the integration of AI, expansion in emerging markets, and collaboration for standardized protocols. However, challenges such as lack of standardization and complex biological responses need to be addressed. Overall, the future of the in-vitro toxicology assays market looks promising, with continuous advancements in technology and a focus on improving safety assessments across various industries.
What are in-vitro toxicology assays?
In-vitro toxicology assays are laboratory tests that assess the toxicity of substances using cell cultures or biological samples. These assays are crucial for evaluating the safety and efficacy of pharmaceuticals, chemicals, and cosmetics without the need for animal testing.
Who are the key players in the in-vitro toxicology assays market?
Key players in the in-vitro toxicology assays market include Thermo Fisher Scientific, Charles River Laboratories, and Eurofins Scientific, among others. These companies are known for their innovative solutions and comprehensive testing services in toxicology.
What are the main drivers of growth in the in-vitro toxicology assays market?
The growth of the in-vitro toxicology assays market is driven by increasing regulatory requirements for safety testing, the rising demand for alternative testing methods, and advancements in technology that enhance assay accuracy and efficiency.
What challenges does the in-vitro toxicology assays market face?
The in-vitro toxicology assays market faces challenges such as the complexity of biological systems that can lead to variability in results, the need for standardization of assays, and the limitations in predicting human responses based on in-vitro data.
What opportunities exist in the in-vitro toxicology assays market?
Opportunities in the in-vitro toxicology assays market include the development of novel assays that can better mimic human physiology, the integration of artificial intelligence for data analysis, and the expansion of applications in personalized medicine and environmental testing.
What trends are shaping the in-vitro toxicology assays market?
Trends in the in-vitro toxicology assays market include the increasing adoption of high-throughput screening technologies, the shift towards more human-relevant models, and the growing emphasis on sustainability in testing practices.
In-vitro Toxicology Assays Market:
| Segmentation | Details |
|---|---|
| Product | Reagents & Kits, Assay Systems, Software |
| Technology | Cell-based Assays, Biochemical Assays, Others |
| Application | Systemic Toxicity Testing, Dermal Toxicity Testing, Others |
| End User | Pharmaceutical & Biotechnology Companies, Contract Research Organizations, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the In-vitro Toxicology Assays Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at