444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The immune checkpoint inhibitor market is experiencing significant growth due to the increasing prevalence of cancer and the rising demand for effective immunotherapy treatments. Immune checkpoint inhibitors are a type of medication that harnesses the body’s immune system to fight cancer by blocking proteins that prevent immune cells from attacking cancer cells. These inhibitors have shown promising results in various types of cancer, leading to their widespread adoption in the medical field.
Meaning
Immune checkpoint inhibitors are a class of drugs that work by releasing the brakes on the immune system, allowing it to recognize and attack cancer cells. The immune system has natural checkpoint proteins that regulate its response to prevent excessive immune activity. However, cancer cells can exploit these checkpoints to avoid detection by the immune system. Immune checkpoint inhibitors, such as PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, block the checkpoints and restore the immune system’s ability to recognize and destroy cancer cells.
Executive Summary
The immune checkpoint inhibitor market is poised for substantial growth in the coming years. The rising incidence of cancer, along with the increasing awareness about immunotherapy treatments, is driving the demand for immune checkpoint inhibitors. These inhibitors have shown impressive results in clinical trials and have become a crucial component of cancer treatment regimens. However, challenges such as high treatment costs and potential side effects need to be addressed to fully unlock the market’s potential.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several key drivers are fueling the growth of the immune checkpoint inhibitor market:
Market Restraints
Despite the positive growth prospects, the immune checkpoint inhibitor market faces certain challenges:
Market Opportunities
The immune checkpoint inhibitor market presents several opportunities for growth and advancement:
Market Dynamics
The immune checkpoint inhibitor market is driven by a combination of factors such as increasing cancer prevalence, advancements in immunotherapy research, and patient demand for effective treatments. The market is highly competitive, with pharmaceutical companies investing heavily in research and development to gain a competitive edge. Strategic collaborations and partnerships are also common in the market, enabling companies to leverage each other’s strengths and resources.
Regional Analysis
The immune checkpoint inhibitor market exhibits regional variations due to differences in cancer prevalence, healthcare infrastructure, and regulatory landscapes. North America leads the market, driven by the high incidence of cancer and the presence of established pharmaceutical companies. Europe follows closely, with several countries adopting immunotherapy in cancer treatment. Asia-Pacific is witnessing significant growth, attributed to improving healthcare infrastructure, rising disposable income, and increasing investments in cancer research.
Competitive Landscape
Leading Companies in the Immune Checkpoint Inhibitor Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

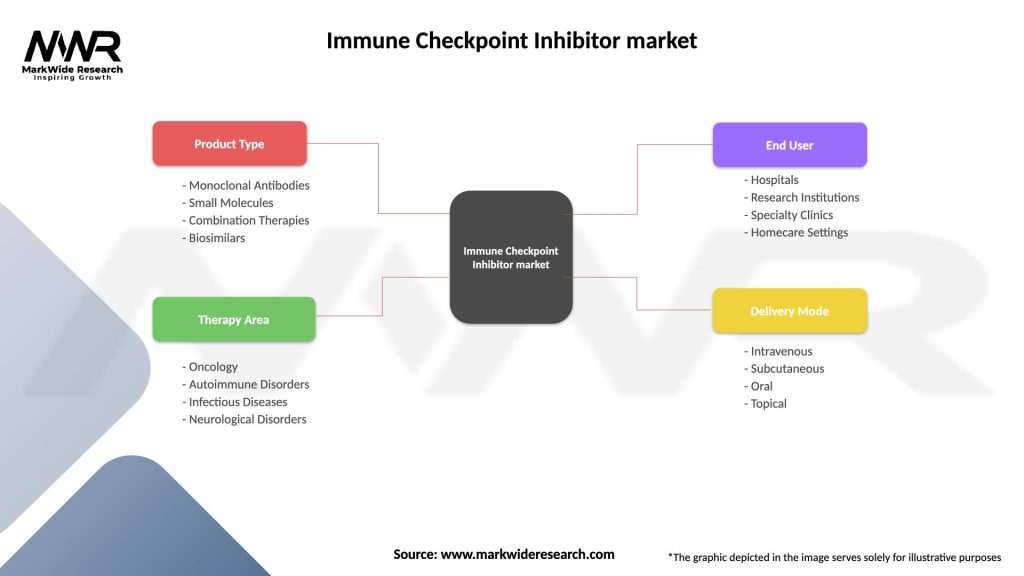
Segmentation
The immune checkpoint inhibitor market can be segmented based on various factors, including:
Segmenting the market helps identify specific target audiences, tailor marketing strategies, and analyze market trends and opportunities.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the immune checkpoint inhibitor market can benefit in the following ways:
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the immune checkpoint inhibitor market. While the pandemic disrupted healthcare systems and clinical trials, the demand for immune checkpoint inhibitors remained steady. Cancer patients still require effective treatment options, and the importance of immunotherapy in cancer care has been emphasized. The pandemic highlighted the need for accessible and personalized treatment options, driving further interest and investment in immune checkpoint inhibitors.
Key Industry Developments
Analyst Suggestions
Based on the current market trends and dynamics, industry analysts suggest the following:
Future Outlook
The future of the immune checkpoint inhibitor market appears promising, driven by advancements in cancer research, the growing prevalence of cancer, and increasing patient demand for effective treatments. The development of novel targets, expansion into emerging markets, and the exploration of combination therapies offer significant growth opportunities. However, addressing challenges related to treatment costs, side effects, and regulatory hurdles will be crucial to fully realize the market’s potential.
Conclusion
The immune checkpoint inhibitor market is witnessing substantial growth, driven by the rising incidence of cancer and the demand for effective immunotherapy treatments. Immune checkpoint inhibitors have revolutionized cancer care by harnessing the body’s immune system to fight cancer cells. While the market presents opportunities for industry participants and stakeholders, challenges such as high treatment costs, potential side effects, and limited efficacy in certain tumor types need to be addressed. Strategic collaborations, research and development, and personalized medicine approaches will shape the future of the immune checkpoint inhibitor market, leading to improved patient outcomes and enhanced cancer treatment options.
What is Immune Checkpoint Inhibitor?
Immune checkpoint inhibitors are a class of drugs that help to enhance the immune system’s response against cancer cells. They work by blocking proteins that inhibit immune responses, allowing T-cells to attack tumors more effectively.
What are the key players in the Immune Checkpoint Inhibitor market?
Key players in the Immune Checkpoint Inhibitor market include Bristol-Myers Squibb, Merck & Co., and Roche, among others. These companies are leading the development and commercialization of various immune checkpoint inhibitors for cancer treatment.
What are the main drivers of the Immune Checkpoint Inhibitor market?
The main drivers of the Immune Checkpoint Inhibitor market include the increasing prevalence of cancer, advancements in immunotherapy, and growing investments in cancer research. These factors contribute to the rising demand for effective cancer treatments.
What challenges does the Immune Checkpoint Inhibitor market face?
The Immune Checkpoint Inhibitor market faces challenges such as high treatment costs, potential side effects, and varying patient responses to therapy. These factors can limit accessibility and effectiveness in certain populations.
What opportunities exist in the Immune Checkpoint Inhibitor market?
Opportunities in the Immune Checkpoint Inhibitor market include the development of combination therapies, expansion into new cancer types, and ongoing clinical trials. These avenues may enhance treatment efficacy and broaden patient access.
What trends are shaping the Immune Checkpoint Inhibitor market?
Trends in the Immune Checkpoint Inhibitor market include the rise of personalized medicine, increased focus on biomarker identification, and the exploration of novel combinations with other therapies. These trends aim to improve patient outcomes and treatment precision.
Immune Checkpoint Inhibitor market
| Segmentation Details | Description |
|---|---|
| Product Type | Monoclonal Antibodies, Small Molecules, Combination Therapies, Biosimilars |
| Therapy Area | Oncology, Autoimmune Disorders, Infectious Diseases, Neurological Disorders |
| End User | Hospitals, Research Institutions, Specialty Clinics, Homecare Settings |
| Delivery Mode | Intravenous, Subcutaneous, Oral, Topical |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Immune Checkpoint Inhibitor Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at