444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview: Exploring the Landscape of the Homocysteine Testing Market
The homocysteine testing market, a crucial segment within the healthcare diagnostics industry, is witnessing substantial growth as awareness about the relationship between elevated homocysteine levels and cardiovascular health gains traction. Homocysteine testing plays a pivotal role in identifying potential risk factors and guiding preventive measures for cardiovascular diseases.
Meaning: Decoding the Significance of Homocysteine Testing
Homocysteine testing involves the measurement of homocysteine levels in blood. Homocysteine is an amino acid produced during the metabolism of methionine, and elevated levels have been linked to an increased risk of cardiovascular diseases. Homocysteine testing aids in assessing cardiovascular health, guiding treatment decisions, and implementing preventive strategies.
Executive Summary: A Glimpse into the Homocysteine Testing Market
The global homocysteine testing market is experiencing notable growth driven by the increasing prevalence of cardiovascular diseases and the growing focus on preventive healthcare. As medical professionals recognize the role of elevated homocysteine levels in cardiovascular risk, the demand for accurate and reliable homocysteine testing methods is on the rise.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
The Homocysteine Testing Market is characterized by several critical insights that define its current landscape and future potential:
These insights indicate a market that is rapidly evolving to meet the diagnostic needs of modern healthcare while driving improvements in patient care and disease prevention.
Market Drivers
Several key factors are propelling the growth of the Homocysteine Testing Market:
These drivers create a favorable environment for the expansion of homocysteine testing as a key component in cardiovascular risk assessment and overall diagnostic services.
Market Restraints
Despite strong growth prospects, the Homocysteine Testing Market faces several challenges:
Addressing these restraints through strategic partnerships, targeted investments in training and infrastructure, and streamlined regulatory processes will be key to sustaining market momentum.
Market Opportunities
The Homocysteine Testing Market presents numerous opportunities for growth and innovation:
By capitalizing on these opportunities, industry stakeholders can broaden their market presence, enhance product offerings, and drive the evolution of early diagnostic strategies.
Market Dynamics
The dynamics of the Homocysteine Testing Market are influenced by a blend of supply-side and demand-side factors as well as broader economic and technological trends:
Supply Side Factors:
Demand Side Factors:
Economic Considerations:
These dynamics illustrate a complex market environment where technological innovation, preventive healthcare trends, and strategic investments converge to drive growth.
Regional Analysis
The Homocysteine Testing Market exhibits distinct trends and growth patterns across various regions:
North America:
Europe:
Asia-Pacific:
Latin America and Middle East & Africa:
Competitive Landscape
Leading Companies in the Homocysteine Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Homocysteine Testing Market can be segmented based on various criteria to provide a detailed understanding of its structure and applications:
By Testing Methodology:
By Application:
By End-User:
By Geography:
This segmentation framework enables market stakeholders to target specific applications and regional needs, tailoring strategies to maximize market penetration.
Category-wise Insights
Each category within the Homocysteine Testing Market offers unique value propositions:
These insights highlight the importance of targeted product development and customized solutions to address the diverse needs of different market segments.
Key Benefits for Industry Participants and Stakeholders
The Homocysteine Testing Market offers several key benefits for manufacturers, healthcare providers, and end-users:
These benefits collectively drive the adoption of homocysteine testing and contribute to improved healthcare outcomes and economic efficiencies.
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Several key trends are shaping the evolution of the Homocysteine Testing Market:
These trends reflect a market in transformation, driven by technological breakthroughs and an increasing focus on preventive healthcare.
Covid-19 Impact
The Covid-19 pandemic has influenced the Homocysteine Testing Market in several significant ways:
These impacts have reinforced the value of homocysteine testing in early disease detection and have accelerated its integration into modern healthcare practices.
Key Industry Developments
The Homocysteine Testing Market has witnessed several notable industry developments:
These developments are not only advancing the technological landscape but also positioning the market for long-term growth and broader clinical acceptance.
Analyst Suggestions
Industry analysts recommend the following strategies for stakeholders in the Homocysteine Testing Market:
Implementing these strategies will enable companies to capitalize on market opportunities, drive technological innovation, and secure a competitive edge in the evolving diagnostic landscape.
Future Outlook
The future outlook for the Homocysteine Testing Market is highly promising, with significant growth anticipated over the next decade. Key factors driving this growth include:
Overall, the Homocysteine Testing Market is set to become a critical component of modern healthcare diagnostics, supporting early disease detection, improved patient outcomes, and cost-effective preventive care.
Conclusion
The Homocysteine Testing Market stands at the forefront of a transformative shift in diagnostic healthcare, driven by the rising prevalence of cardiovascular and metabolic disorders and the global move toward preventive medicine. By enabling early detection and personalized treatment strategies, homocysteine testing plays a vital role in reducing disease risks and improving long-term patient outcomes. Despite challenges such as high capital investment and regulatory complexities, continuous technological advancements, digital integration, and strategic collaborations are positioning the market for robust growth. Stakeholders that invest in advanced R&D, expand their geographic footprint, and embrace digital transformation will be well-equipped to capture emerging opportunities and contribute to a more proactive, efficient, and patient-centric healthcare ecosystem.
What is Homocysteine Testing?
Homocysteine Testing refers to the measurement of homocysteine levels in the blood, which is important for assessing cardiovascular health and the risk of certain diseases. Elevated homocysteine levels can indicate deficiencies in vitamins B6, B12, and folate, among other health issues.
What are the key players in the Homocysteine Testing market?
Key players in the Homocysteine Testing market include Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers, among others. These companies are involved in developing and providing diagnostic tests and equipment for measuring homocysteine levels.
What are the growth factors driving the Homocysteine Testing market?
The growth of the Homocysteine Testing market is driven by increasing awareness of cardiovascular diseases, the rising prevalence of conditions associated with elevated homocysteine levels, and advancements in diagnostic technologies. Additionally, the growing focus on preventive healthcare is contributing to market expansion.
What challenges does the Homocysteine Testing market face?
The Homocysteine Testing market faces challenges such as the lack of standardization in testing methods and the need for further research to establish clear clinical guidelines. Additionally, the high cost of advanced testing equipment can limit accessibility in some regions.
What opportunities exist in the Homocysteine Testing market?
Opportunities in the Homocysteine Testing market include the development of point-of-care testing devices and the integration of homocysteine testing into routine health check-ups. Furthermore, increasing collaborations between diagnostic companies and healthcare providers can enhance market reach.
What trends are emerging in the Homocysteine Testing market?
Emerging trends in the Homocysteine Testing market include the use of digital health technologies for remote monitoring and the growing emphasis on personalized medicine. Additionally, there is an increasing trend towards the incorporation of homocysteine testing in comprehensive metabolic panels.
Homocysteine Testing market
| Segmentation Details | Description |
|---|---|
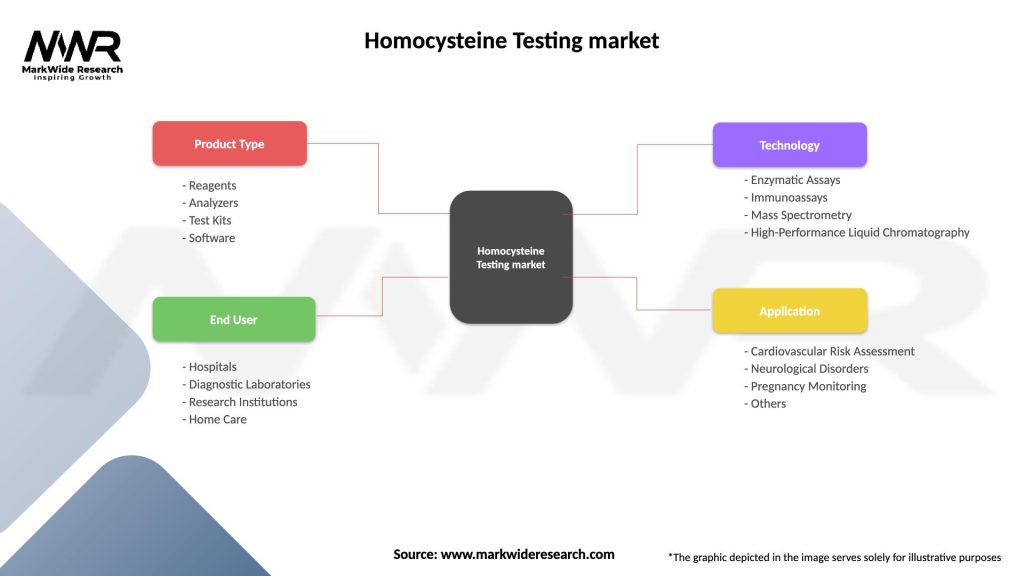
| Product Type | Reagents, Analyzers, Test Kits, Software |
| End User | Hospitals, Diagnostic Laboratories, Research Institutions, Home Care |
| Technology | Enzymatic Assays, Immunoassays, Mass Spectrometry, High-Performance Liquid Chromatography |
| Application | Cardiovascular Risk Assessment, Neurological Disorders, Pregnancy Monitoring, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Homocysteine Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at