444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The HIV clinical trials market is a crucial segment of the healthcare industry that focuses on researching and developing new treatments, therapies, and interventions for individuals living with HIV/AIDS. These clinical trials play a vital role in advancing medical knowledge, improving patient outcomes, and ultimately finding a cure for this devastating disease. The market for HIV clinical trials encompasses a wide range of stakeholders, including pharmaceutical companies, research organizations, academic institutions, government agencies, and healthcare providers.
Meaning
HIV, which stands for Human Immunodeficiency Virus, is a viral infection that attacks the immune system, making it difficult for the body to fight off infections and diseases. Acquired Immunodeficiency Syndrome (AIDS) is the advanced stage of HIV infection, characterized by severe immune system damage. HIV clinical trials refer to the systematic investigations conducted to evaluate the safety, efficacy, and tolerability of new drugs, therapies, vaccines, and preventive measures for HIV/AIDS. These trials are conducted on human subjects under controlled conditions to gather data and evidence necessary for regulatory approval and clinical practice.
Executive Summary
The HIV clinical trials market has witnessed significant growth and advancements over the years, driven by the need for innovative treatments, rising HIV prevalence globally, and increasing investments in research and development. The market is highly competitive, with numerous players striving to develop breakthrough interventions and gain a competitive edge. However, the market also faces various challenges, such as stringent regulatory requirements, ethical considerations, and the complexity of HIV/AIDS as a disease. Despite these challenges, the HIV clinical trials market continues to expand, offering hope for improved therapies and a potential cure for HIV/AIDS.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The HIV clinical trials market is characterized by constant innovation, evolving regulatory landscapes, and dynamic collaborations among stakeholders. Factors such as increasing HIV prevalence, advancements in antiretroviral therapy, government support, and rising awareness contribute to market growth. However, regulatory challenges, high research costs, patient recruitment issues, and long trial durations pose hurdles to market expansion. Opportunities lie in the development of long-acting therapies, combination treatments, novel preventive strategies, and targeted interventions for pediatric HIV/AIDS.
Regional Analysis
The HIV clinical trials market exhibits regional variations influenced by factors such as HIV prevalence, healthcare infrastructure, research capabilities, and regulatory environments. Regions heavily impacted by the HIV epidemic, such as sub-Saharan Africa, have a significant presence of clinical trials focused on evaluating interventions relevant to their population. Developed regions, such as North America and Europe, contribute to research advancements and play a crucial role in global collaborations. Asia-Pacific and Latin American regions offer growth opportunities due to increasing investments in healthcare infrastructure and research capabilities.
Competitive Landscape
Leading Companies in the HIV Clinical Trials Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

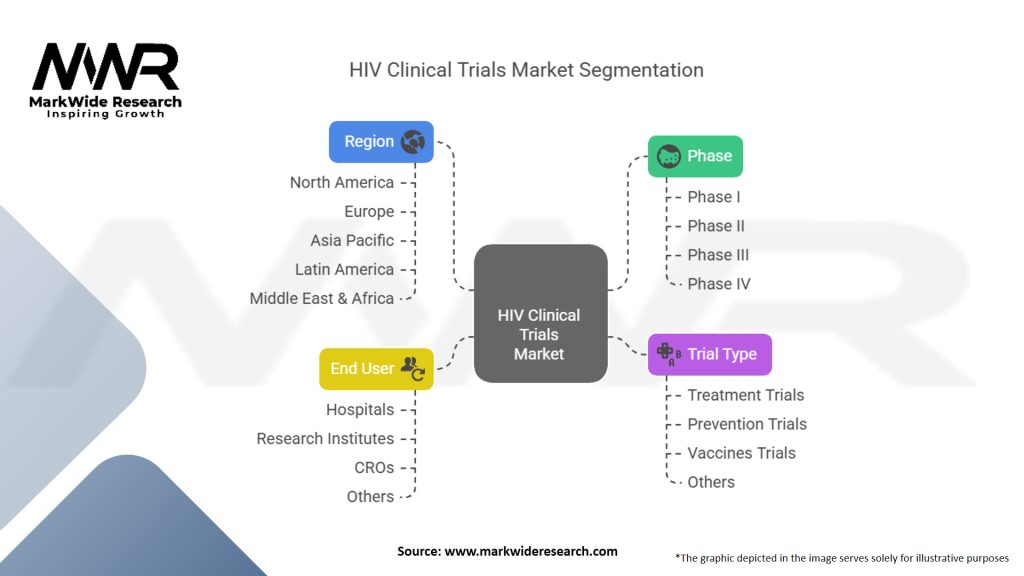
Segmentation
The HIV clinical trials market can be segmented based on various criteria, including intervention type, trial phase, target population, and geographic region. Intervention types may include antiretroviral drugs, vaccines, immunotherapies, gene therapies, and preventive measures. Trial phases typically range from Phase I (safety evaluation) to Phase III (efficacy evaluation), with some interventions progressing to Phase IV (post-marketing surveillance). Target populations can encompass adult patients, pediatric patients, treatment-experienced individuals, and specific demographic or risk groups.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths
Weaknesses
Opportunities
Threats
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the HIV clinical trials market. The diversion of healthcare resources and disruptions in research activities due to the pandemic has affected the progress of ongoing trials and delayed the initiation of new trials. Clinical trial protocols have been modified to ensure participant safety and adherence to infection control measures. Virtual trial visits, remote monitoring, and telemedicine have been implemented to minimize in-person interactions. Despite these challenges, the pandemic has also led to increased collaboration, resource sharing, and accelerated research efforts to find synergies between HIV/AIDS and COVID-19 research.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the HIV clinical trials market is promising, with continued advancements in research, technology, and collaborative efforts. The development of long-acting therapies, combination treatments, novel preventive strategies, and targeted interventions for specific populations will shape the market. Personalized medicine approaches, cure research, and the integration of digital health solutions will revolutionize the field. Overcoming regulatory challenges, ensuring patient-centric approaches, and strengthening global research networks will be critical for future success.
Conclusion
The HIV clinical trials market plays a vital role in advancing knowledge, improving patient outcomes, and finding a cure for HIV/AIDS. Despite challenges such as regulatory complexities, high research costs, and patient recruitment issues, the market continues to grow due to increasing HIV prevalence, research investments, and technological advancements. Opportunities lie in the development of long-acting therapies, combination treatments, novel preventive strategies, and targeted interventions for pediatric HIV/AIDS. The market is characterized by collaborations, regional variations, and a highly competitive landscape. The future outlook is promising, driven by research advancements, personalized medicine approaches, and the integration of digital health solutions. The ultimate goal remains improving patient outcomes and finding a cure for HIV/AIDS.
What are HIV clinical trials?
HIV clinical trials are research studies that evaluate new treatments, therapies, or interventions for HIV infection. These trials aim to improve patient outcomes and advance the understanding of HIV-related health issues.
Who are the key players in the HIV clinical trials market?
Key players in the HIV clinical trials market include pharmaceutical companies such as Gilead Sciences, Merck & Co., and ViiV Healthcare, which are actively involved in developing innovative therapies for HIV, among others.
What are the main drivers of growth in the HIV clinical trials market?
The main drivers of growth in the HIV clinical trials market include the increasing prevalence of HIV, advancements in treatment technologies, and a growing focus on personalized medicine. Additionally, rising funding for HIV research contributes to market expansion.
What challenges does the HIV clinical trials market face?
The HIV clinical trials market faces challenges such as regulatory hurdles, patient recruitment difficulties, and the high costs associated with conducting trials. These factors can hinder the timely development of new therapies.
What opportunities exist in the HIV clinical trials market?
Opportunities in the HIV clinical trials market include the potential for novel therapeutic approaches, such as gene therapy and long-acting injectable treatments. Additionally, increasing collaboration between public and private sectors can enhance research efforts.
What trends are shaping the HIV clinical trials market?
Trends shaping the HIV clinical trials market include the rise of digital health technologies for patient monitoring and data collection, as well as a shift towards adaptive trial designs that allow for more flexible and efficient study protocols.
HIV Clinical Trials Market
| Segmentation Details | Information |
|---|---|
| Phase | Phase I, Phase II, Phase III, Phase IV |
| Trial Type | Treatment Trials, Prevention Trials, Vaccines Trials, Others |
| End User | Hospitals, Research Institutes, Contract Research Organizations (CROs), Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the HIV Clinical Trials Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at