444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Gonorrhea Diagnostics market is witnessing significant growth due to the increasing prevalence of gonorrhea infections globally. Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is a sexually transmitted infection (STI) that affects both men and women. Early diagnosis and appropriate treatment are crucial to prevent the spread of the disease and its complications. The market for gonorrhea diagnostics comprises various diagnostic tests, including nucleic acid amplification tests (NAATs), point-of-care (POC) tests, and serological tests.
Meaning
Gonorrhea diagnostics refer to the procedures and tests conducted to detect the presence of Neisseria gonorrhoeae bacteria in patients suspected of having gonorrhea. These diagnostic tests help healthcare professionals accurately identify and diagnose gonorrhea, enabling timely treatment and prevention of further transmission.
Executive Summary
The gonorrhea diagnostics market is experiencing substantial growth, primarily driven by the increasing incidence of gonorrhea infections worldwide. The market is witnessing the development and adoption of advanced diagnostic technologies, such as NAATs and POC tests, which offer high accuracy and rapid results. Additionally, the rising awareness regarding the importance of early detection and treatment of gonorrhea is contributing to market growth.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
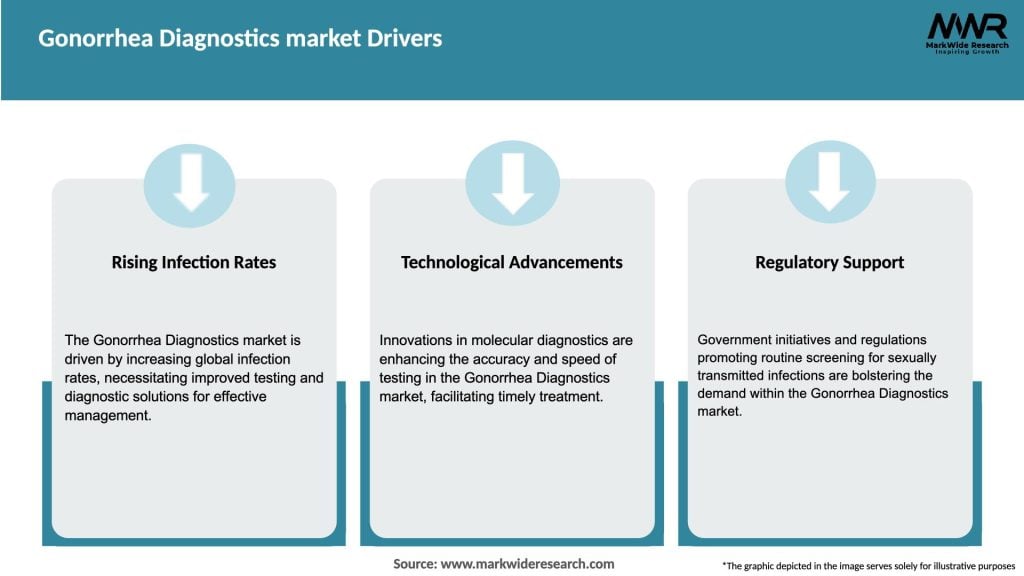
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The gonorrhea diagnostics market is driven by several factors, including the increasing prevalence of gonorrhea infections, technological advancements in diagnostic tests, and growing awareness about STIs. The market is also influenced by regulatory guidelines, healthcare infrastructure development, and social factors such as stigma. Continuous research and development efforts are being made to improve the accuracy, speed, and accessibility of gonorrhea diagnostic tests.
Regional Analysis
The gonorrhea diagnostics market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America and Europe hold significant market shares due to the high prevalence of gonorrhea infections and well-established healthcare infrastructure. Asia Pacific is expected to witness substantial growth due to the increasing awareness about STIs, rising healthcare investments, and the large population base.
Competitive Landscape
Leading Companies in the Gonorrhea Diagnostics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The gonorrhea diagnostics market is segmented based on test type, end-user, and region. By test type, the market can be divided into nucleic acid amplification tests (NAATs), point-of-care (POC) tests, and serological tests. The end-users of these diagnostic tests include hospitals, diagnostic laboratories, and clinics.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on healthcare systems globally, diverting resources and attention away from STI testing and diagnosis, including gonorrhea. However, as the situation improves, healthcare authorities are expected to reinstate and strengthen STI testing programs, including gonorrhea diagnostics. The pandemic has also highlighted the importance of rapid and accurate diagnostic tests, leading to advancements in technology and the adoption of innovative approaches in gonorrhea diagnostics.
Key Industry Developments
Advancements in Molecular Diagnostics: New innovations in nucleic acid amplification tests (NAATs) have enhanced the accuracy and speed of Gonorrhea detection, allowing for more reliable results in a shorter timeframe.
Growth of Point-of-Care Testing: The increasing demand for point-of-care diagnostic solutions has led to the development of portable, easy-to-use testing kits that provide rapid results, especially in remote and underserved regions.
Integration of Digital Health: The rise of digital health platforms, including mobile applications and telemedicine, has facilitated remote consultations and monitoring, expanding the reach of Gonorrhea testing.
Collaborations and Partnerships: Public health organizations and diagnostic companies are forming strategic partnerships to improve STI testing access, with a focus on increasing awareness and availability of Gonorrhea diagnostic tools in low-resource settings.
Analyst Suggestions
1.Healthcare organizations and diagnostic companies should focus on research and development to introduce more accurate, rapid, and user-friendly gonorrhea diagnostic tests. 2. Increased collaboration between diagnostic companies, healthcare organizations, and government bodies can help raise awareness about gonorrhea, promote testing, and implement comprehensive STI screening programs.
Future Outlook
The gonorrhea diagnostics market is projected to witness substantial growth in the coming years. Factors such as increasing awareness about STIs, technological advancements in diagnostic tests, and the implementation of comprehensive STI screening programs will contribute to market expansion. The development of novel diagnostic technologies and the focus on point-of-care testing are expected to drive market growth. However, regulatory challenges and the need for skilled professionals may pose some limitations.
Conclusion
The gonorrhea diagnostics market is experiencing significant growth due to the increasing prevalence of gonorrhea infections and the need for early diagnosis and treatment. The market is driven by technological advancements, growing awareness, and initiatives to control the spread of STIs. Nucleic acid amplification tests (NAATs) and point-of-care (POC) tests are the key diagnostic methods, offering accuracy and rapid results. Regional expansion, collaborations, and continuous research and development efforts are expected to shape the future of the gonorrhea diagnostics market.
What is Gonorrhea Diagnostics?
Gonorrhea Diagnostics refers to the methods and technologies used to detect the presence of the Neisseria gonorrhoeae bacteria, which causes gonorrhea. These diagnostics are crucial for timely treatment and prevention of complications associated with the infection.
What are the key players in the Gonorrhea Diagnostics market?
Key players in the Gonorrhea Diagnostics market include Abbott Laboratories, Roche Diagnostics, and Hologic, among others. These companies are known for their innovative diagnostic solutions and contribute significantly to advancements in testing methodologies.
What are the main drivers of growth in the Gonorrhea Diagnostics market?
The growth of the Gonorrhea Diagnostics market is driven by increasing rates of gonorrhea infections, rising awareness about sexually transmitted infections, and advancements in diagnostic technologies. Additionally, government initiatives to promote sexual health are also contributing to market expansion.
What challenges does the Gonorrhea Diagnostics market face?
The Gonorrhea Diagnostics market faces challenges such as the emergence of antibiotic-resistant strains of Neisseria gonorrhoeae, which complicates treatment and diagnosis. Furthermore, limited access to healthcare facilities in certain regions can hinder effective testing and management.
What opportunities exist in the Gonorrhea Diagnostics market?
Opportunities in the Gonorrhea Diagnostics market include the development of rapid and point-of-care testing solutions, which can enhance accessibility and convenience for patients. Additionally, increasing investment in research and development for innovative diagnostic tools presents significant growth potential.
What trends are shaping the Gonorrhea Diagnostics market?
Trends in the Gonorrhea Diagnostics market include the integration of molecular diagnostics and digital health technologies, which improve accuracy and speed of testing. There is also a growing emphasis on personalized medicine and tailored treatment approaches based on diagnostic results.
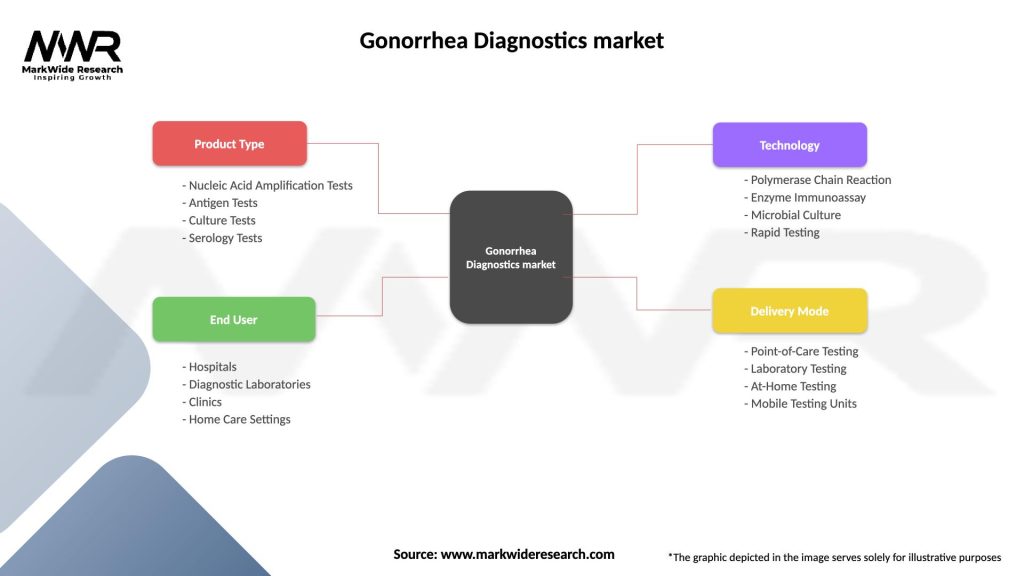
Gonorrhea Diagnostics market
| Segmentation Details | Description |
|---|---|
| Product Type | Nucleic Acid Amplification Tests, Antigen Tests, Culture Tests, Serology Tests |
| End User | Hospitals, Diagnostic Laboratories, Clinics, Home Care Settings |
| Technology | Polymerase Chain Reaction, Enzyme Immunoassay, Microbial Culture, Rapid Testing |
| Delivery Mode | Point-of-Care Testing, Laboratory Testing, At-Home Testing, Mobile Testing Units |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Gonorrhea Diagnostics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at