444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Global Quadrivalent Flu Vaccine market is a thriving segment of the pharmaceutical industry focused on preventing influenza infections. The market encompasses the development, production, and distribution of vaccines that provide protection against four strains of influenza viruses. These vaccines are formulated to target two influenza A viruses and two influenza B viruses, offering a broader spectrum of coverage compared to traditional trivalent vaccines. The Global Quadrivalent Flu Vaccine market plays a crucial role in public health, as it helps reduce the burden of seasonal influenza and its associated complications.
Meaning
Quadrivalent flu vaccines are designed to provide protection against four different strains of influenza viruses, including two influenza A strains and two influenza B strains. The inclusion of two influenza B strains is a key differentiating factor from trivalent vaccines, which only target one influenza B strain. By incorporating a broader range of strains, quadrivalent flu vaccines offer enhanced protection and help reduce the risk of influenza-related illness and hospitalizations. These vaccines are formulated based on the World Health Organization’s recommendations for the prevalent strains of influenza each year.
Executive Summary
The executive summary provides a concise overview of the global quadrivalent flu vaccine market, summarizing the key points and insights discussed in the report. It highlights the market size, growth rate, major market players, and key trends driving the market. The executive summary serves as a snapshot of the market, providing readers with a quick understanding of the market’s current status and future prospects.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several factors are driving the growth of the Global Quadrivalent Flu Vaccine Market:
Market Restraints
Despite the promising growth, several challenges exist in the Global Quadrivalent Flu Vaccine Market:
Market Opportunities
The Global Quadrivalent Flu Vaccine Market presents several opportunities for growth:
Market Dynamics
The Global Quadrivalent Flu Vaccine Market is influenced by several key dynamics:
Regional Analysis
The Global Quadrivalent Flu Vaccine Market shows varying demand by region:
Competitive Landscape
Leading Companies in Global Quadrivalent Flu Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
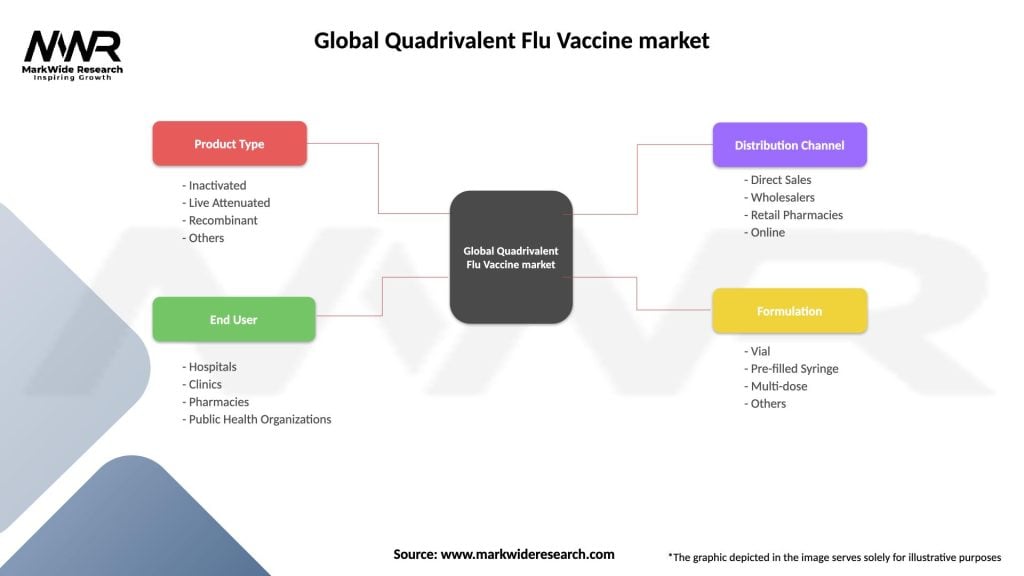
The Global Quadrivalent Flu Vaccine Market can be segmented based on the following factors:
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The Covid-19 impact section evaluates the influence of the pandemic on the global quadrivalent flu vaccine market. It discusses the interplay between influenza and Covid-19, highlighting the importance of maintaining high vaccination rates to mitigate the impact of both diseases. The section explores the challenges and opportunities faced by the market during the pandemic, such as disruptions in vaccine supply chains, changes in healthcare-seeking behavior, and increased public awareness of respiratory illnesses. It also examines the strategies adopted by market players to adapt to the Covid-19 pandemic and ensure vaccine availability.
Key Industry Developments
The key industry developments section highlights recent advancements, initiatives, and collaborations in the global quadrivalent flu vaccine market. It covers topics such as new product launches, regulatory approvals, research and development activities, and strategic partnerships. The section provides insights into the latest developments that are shaping the market landscape and driving innovation in vaccine manufacturing, distribution, and administration.
Analyst Suggestions
The analyst suggestions section offers recommendations and guidance for industry participants and stakeholders in the global quadrivalent flu vaccine market. It provides insights into strategies for market entry, product differentiation, and competitive positioning. The section emphasizes the importance of continuous research and development efforts to enhance vaccine efficacy, safety, and convenience. It also encourages collaborations between vaccine manufacturers, healthcare providers, and government agencies to strengthen vaccination programs and increase vaccine accessibility.
Future Outlook
The future outlook section provides a forward-looking perspective on the global quadrivalent flu vaccine market. It discusses anticipated market trends, technological advancements, regulatory developments, and evolving consumer preferences that are likely to shape the market in the coming years. The section highlights potential growth opportunities in emerging markets, the impact of evolving healthcare policies, and the integration of digital health solutions in vaccine delivery. It emphasizes the importance of proactive measures to ensure vaccine availability and accessibility for global population health.
Conclusion
In conclusion, the global quadrivalent flu vaccine market plays a crucial role in preventing and controlling influenza by providing broader protection against multiple strains of the virus. The market is driven by the increasing prevalence of influenza, growing awareness of vaccination, and advancements in vaccine manufacturing technology. However, challenges such as high production costs, limited access to vaccines, and vaccine hesitancy pose restraints to market growth. The market offers significant opportunities for expansion, including untapped markets, combination vaccines, and novel delivery methods. Strategic collaborations, research and development efforts, and regulatory support are essential for industry participants and stakeholders to capitalize on these opportunities and address the evolving needs of the global population. With the ongoing impact of Covid-19, maintaining high vaccination rates and strengthening global vaccination programs are crucial for preventing the simultaneous spread of influenza and Covid-19. The future outlook for the global quadrivalent flu vaccine market is optimistic, with the potential for continued advancements in vaccine technology, increased vaccination coverage, and improved public health outcomes.
What is Quadrivalent Flu Vaccine?
Quadrivalent Flu Vaccine is a type of influenza vaccine designed to protect against four different strains of the flu virus, typically two A strains and two B strains. This vaccine aims to provide broader protection compared to trivalent vaccines, which cover only three strains.
What are the key players in the Global Quadrivalent Flu Vaccine market?
Key players in the Global Quadrivalent Flu Vaccine market include Sanofi Pasteur, GlaxoSmithKline, Seqirus, and Pfizer, among others. These companies are involved in the research, development, and distribution of flu vaccines worldwide.
What are the growth factors driving the Global Quadrivalent Flu Vaccine market?
The growth of the Global Quadrivalent Flu Vaccine market is driven by increasing awareness of influenza prevention, rising vaccination rates, and the ongoing development of more effective vaccines. Additionally, seasonal flu outbreaks and public health initiatives contribute to the demand for these vaccines.
What challenges does the Global Quadrivalent Flu Vaccine market face?
The Global Quadrivalent Flu Vaccine market faces challenges such as vaccine hesitancy among populations, variations in flu virus strains, and the need for continuous updates to vaccine formulations. These factors can impact vaccination rates and overall effectiveness.
What opportunities exist in the Global Quadrivalent Flu Vaccine market?
Opportunities in the Global Quadrivalent Flu Vaccine market include advancements in vaccine technology, such as mRNA vaccines, and expanding vaccination programs in developing regions. Additionally, increasing government support for immunization initiatives presents further growth potential.
What trends are shaping the Global Quadrivalent Flu Vaccine market?
Trends shaping the Global Quadrivalent Flu Vaccine market include the integration of digital health technologies for vaccine tracking and education, as well as a growing emphasis on personalized medicine. Furthermore, collaborations between public health organizations and vaccine manufacturers are becoming more common.
Global Quadrivalent Flu Vaccine market
| Segmentation Details | Description |
|---|---|
| Product Type | Inactivated, Live Attenuated, Recombinant, Others |
| End User | Hospitals, Clinics, Pharmacies, Public Health Organizations |
| Distribution Channel | Direct Sales, Wholesalers, Retail Pharmacies, Online |
| Formulation | Vial, Pre-filled Syringe, Multi-dose, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Global Quadrivalent Flu Vaccine Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at