444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The global orphan drugs market is a rapidly growing sector within the pharmaceutical industry. Orphan drugs are medications specifically developed to treat rare diseases or conditions that affect a small number of individuals. These diseases often have a limited patient population, making it challenging for pharmaceutical companies to invest in research and development. However, the increasing prevalence of rare diseases and favorable regulatory incentives have driven the growth of the orphan drugs market.
Orphan drugs are pharmaceutical products developed to treat rare diseases or conditions that affect a small portion of the population. These diseases are often life-threatening or chronically debilitating, and there is a significant unmet medical need for effective treatments. Due to the limited patient population, developing orphan drugs can be financially challenging for pharmaceutical companies without regulatory support.
Executive Summary
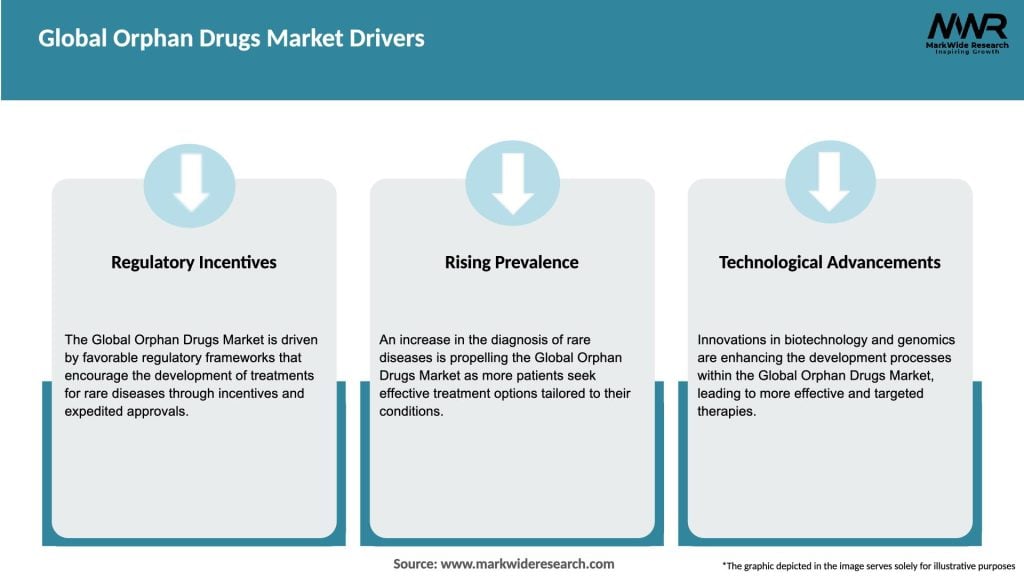
The global orphan drugs market has witnessed substantial growth in recent years. The market is driven by factors such as increasing prevalence of rare diseases, favorable government regulations, financial incentives, and advancements in biotechnology. Despite the challenges associated with developing orphan drugs, the market presents significant opportunities for pharmaceutical companies willing to invest in this niche sector.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The orphan drugs market operates within a complex set of dynamics influenced by various factors:
Regional Analysis
The orphan drugs market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory frameworks, and disease prevalence.
Competitive Landscape
Leading Companies in the Global Orphan Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
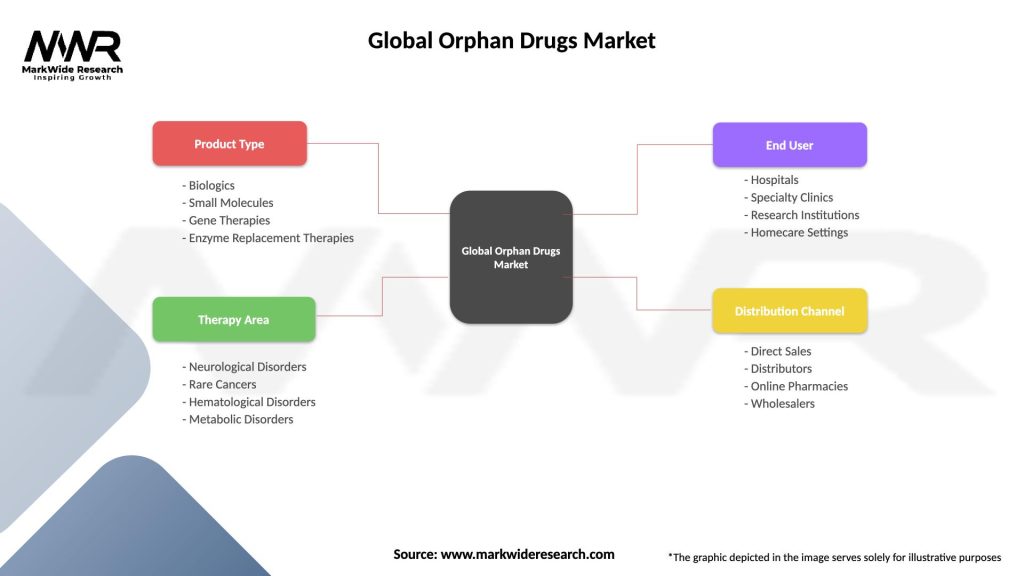
Segmentation
The orphan drugs market can be segmented based on various factors such as disease type, therapeutic class, and region. Common segments include:
Segmentation helps in understanding the specific needs and requirements of different disease categories and therapeutic classes. It enables pharmaceutical companies to target their research, development, and marketing efforts effectively.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT analysis helps identify the strengths, weaknesses, opportunities, and threats in the orphan drugs market.
Strengths:
Weaknesses:
Opportunities:
Threats:
Conducting a thorough SWOT analysis allows stakeholders to understand the internal strengths and weaknesses of their organizations and identify external opportunities and threats in the orphan drugs market.
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had both direct and indirect impacts on the orphan drugs market:
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the global orphan drugs market appears promising, with several key trends and developments driving growth. Advancements in technology, increasing research collaborations, and a focus on personalized medicine are set to reshape the orphan drugs landscape. The ongoing COVID-19 pandemic has highlighted the importance of rare diseases and may lead to increased investments and regulatory support for orphan drug research. With the growing prevalence of rare diseases and favorable regulatory incentives, the orphan drugs market is expected to witness sustained growth in the coming years.
Conclusion
The global orphan drugs market holds immense potential for pharmaceutical companies willing to invest in the development of treatments for rare diseases. The market offers opportunities for expansion, driven by factors such as increasing disease prevalence, favorable government regulations, and advancements in biotechnology. However, challenges related to high development costs, pricing and reimbursement, and limited patient populations persist. By embracing technological innovations, adopting patient-centric approaches, and fostering collaboration, stakeholders can overcome these challenges and thrive in the orphan drugs market. With a focus on personalized medicine, accelerated drug development, and strategic industry developments, the future outlook for the orphan drugs market is promising, paving the way for improved treatments and better outcomes for patients with rare diseases.
What is Orphan Drugs?
Orphan drugs are medications developed specifically to treat rare diseases or conditions that affect a small percentage of the population. These drugs often face unique challenges in development and marketing due to their limited patient base.
What are the key players in the Global Orphan Drugs Market?
Key players in the Global Orphan Drugs Market include companies like Novartis, Gilead Sciences, and Amgen, which are known for their innovative treatments for rare diseases. These companies focus on research and development to address unmet medical needs, among others.
What are the main drivers of growth in the Global Orphan Drugs Market?
The growth of the Global Orphan Drugs Market is driven by factors such as increasing prevalence of rare diseases, advancements in biotechnology, and favorable regulatory policies that encourage the development of orphan drugs. Additionally, rising awareness among healthcare providers and patients contributes to market expansion.
What challenges does the Global Orphan Drugs Market face?
The Global Orphan Drugs Market faces challenges including high development costs, limited patient populations for clinical trials, and complex regulatory requirements. These factors can hinder the timely availability of new treatments for rare diseases.
What opportunities exist in the Global Orphan Drugs Market?
Opportunities in the Global Orphan Drugs Market include the potential for partnerships between pharmaceutical companies and research institutions, as well as the development of personalized medicine approaches. Additionally, increasing investment in rare disease research presents significant growth potential.
What trends are shaping the Global Orphan Drugs Market?
Trends shaping the Global Orphan Drugs Market include the rise of gene therapies, advancements in precision medicine, and the growing use of digital health technologies to monitor patient outcomes. These innovations are transforming how orphan diseases are treated and managed.
Global Orphan Drugs Market
| Segmentation Details | Description |
|---|---|
| Product Type | Biologics, Small Molecules, Gene Therapies, Enzyme Replacement Therapies |
| Therapy Area | Neurological Disorders, Rare Cancers, Hematological Disorders, Metabolic Disorders |
| End User | Hospitals, Specialty Clinics, Research Institutions, Homecare Settings |
| Distribution Channel | Direct Sales, Distributors, Online Pharmacies, Wholesalers |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Global Orphan Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at