444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The global in silico clinical trials market is witnessing significant growth, driven by advancements in technology and the need to streamline the drug development process. In silico clinical trials refer to the use of computer simulation and modeling techniques to predict the safety and efficacy of new drugs and medical treatments. This comprehensive analysis provides insights into the current state and future prospects of the global in silico clinical trials market.
Meaning
In silico clinical trials are virtual trials conducted using computer simulations and modeling techniques. These trials enable researchers and pharmaceutical companies to predict the effects of new drugs and treatments without the need for traditional human or animal testing. In silico clinical trials offer numerous advantages, including reduced costs, faster time-to-market, and enhanced safety assessment.
Executive Summary
The executive summary offers a concise overview of the key findings and highlights of the global in silico clinical trials market. It outlines the market size, growth rate, and major trends observed in the industry. The summary also includes a snapshot of the competitive landscape and key recommendations for industry participants and stakeholders.
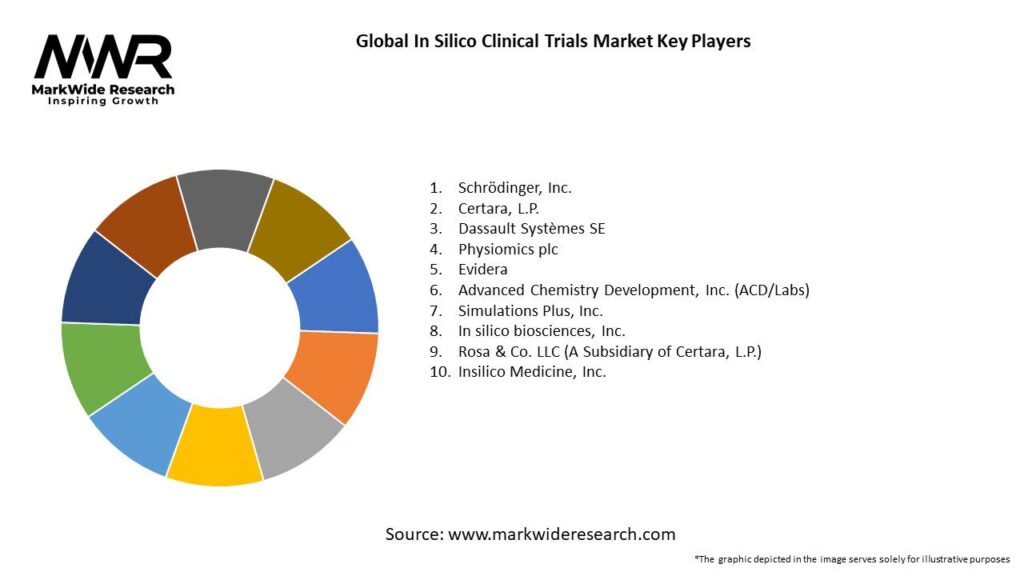
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
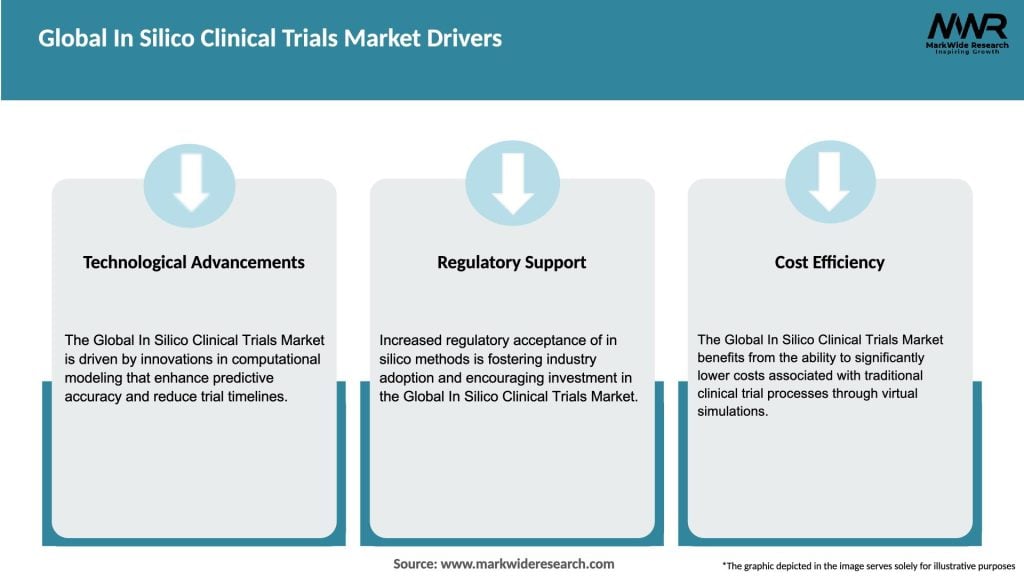
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
Regional Analysis
The in silico clinical trials market is seeing strong growth in North America and Europe, where there is a well-established healthcare infrastructure, high levels of investment in research and development, and increasing regulatory acceptance. In these regions, pharmaceutical companies and biotech firms are increasingly adopting in silico trials to streamline drug development processes.
The Asia-Pacific region, particularly China and India, is expected to see rapid growth in the coming years, driven by rising healthcare investments, increased demand for affordable healthcare solutions, and growing participation in clinical trials. As the region’s healthcare infrastructure continues to improve, in silico trials will play a crucial role in accelerating drug discovery and development.
Competitive Landscape
Leading Companies in Global In Silico Clinical Trials Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
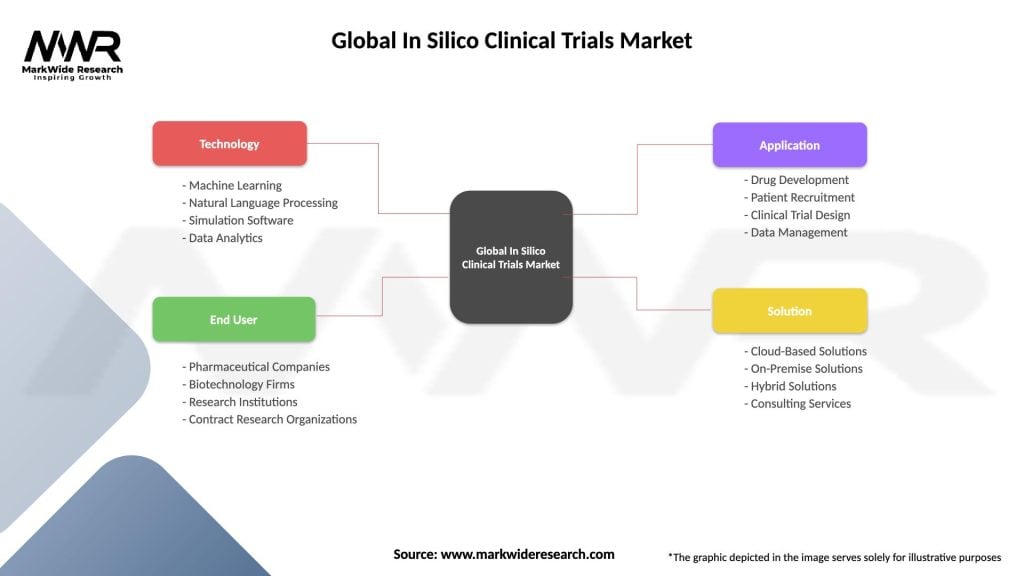
Segmentation
By Technology
By End-User
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 impact section examines the repercussions of the pandemic on the global in silico clinical trials market. It discusses the short-term and long-term effects, including the acceleration of digital transformation initiatives, the increased reliance on virtual trials, and the role of in silico modeling in vaccine development. The section offers insights into the market’s resilience and adaptation strategies.
Key Industry Developments
The key industry developments section highlights the recent advancements and innovations in the global in silico clinical trials market. It covers partnerships, collaborations, research and development activities, and regulatory updates. The section provides readers with up-to-date information on the market’s latest developments.
Analyst Suggestions
The analyst suggestions section offers expert recommendations and strategies for industry participants and stakeholders in the global in silico clinical trials market. It provides actionable insights to help companies adopt in silico approaches, navigate regulatory requirements, and foster collaboration between academia, industry, and regulatory bodies. The section assists stakeholders in making informed decisions and staying ahead of the competition.
Future Outlook
The future outlook section provides a comprehensive analysis of the global in silico clinical trials market’s growth prospects and opportunities. It considers factors such as the increasing adoption of in silico approaches by regulatory agencies, the integration of real-world data in modeling and simulation, and the potential for personalized medicine. The section helps stakeholders formulate effective strategies for long-term success.
Conclusion
The conclusion summarizes the key findings and insights discussed throughout the analysis of the global in silico clinical trials market. It reiterates the market’s growth potential, highlights the major trends and challenges, and emphasizes the transformative impact of in silico approaches on the drug development process. The conclusion serves as a comprehensive overview of the market for readers seeking a summary of the report’s key points.
What is In Silico Clinical Trials?
In Silico Clinical Trials refer to the use of computer simulations and models to predict the outcomes of clinical trials. This approach allows researchers to analyze complex biological systems and drug interactions without the need for extensive physical testing.
What are the key players in the Global In Silico Clinical Trials Market?
Key players in the Global In Silico Clinical Trials Market include companies like Certara, Simulations Plus, and Dassault Systèmes. These companies are known for their innovative software solutions and platforms that facilitate virtual clinical trials, among others.
What are the main drivers of growth in the Global In Silico Clinical Trials Market?
The growth of the Global In Silico Clinical Trials Market is driven by factors such as the increasing demand for cost-effective drug development, advancements in computational technologies, and the need for faster clinical trial processes. Additionally, regulatory support for digital solutions is enhancing market expansion.
What challenges does the Global In Silico Clinical Trials Market face?
The Global In Silico Clinical Trials Market faces challenges such as regulatory hurdles, the need for validation of simulation models, and concerns regarding data privacy and security. These factors can hinder the widespread adoption of in silico methods in clinical research.
What opportunities exist in the Global In Silico Clinical Trials Market?
Opportunities in the Global In Silico Clinical Trials Market include the potential for personalized medicine, the integration of artificial intelligence in simulations, and the expansion of partnerships between technology firms and pharmaceutical companies. These trends can lead to more efficient and targeted clinical trials.
What are the current trends in the Global In Silico Clinical Trials Market?
Current trends in the Global In Silico Clinical Trials Market include the increasing use of machine learning algorithms, the development of patient-specific models, and the growing emphasis on real-world evidence. These innovations are shaping the future of clinical trial methodologies.
Global In Silico Clinical Trials Market
| Segmentation Details | Description |
|---|---|
| Technology | Machine Learning, Natural Language Processing, Simulation Software, Data Analytics |
| End User | Pharmaceutical Companies, Biotechnology Firms, Research Institutions, Contract Research Organizations |
| Application | Drug Development, Patient Recruitment, Clinical Trial Design, Data Management |
| Solution | Cloud-Based Solutions, On-Premise Solutions, Hybrid Solutions, Consulting Services |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Global In Silico Clinical Trials Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at