444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing market refers to the market for diagnostic tests designed to detect the presence of H. pylori bacteria in the human body without the need for invasive procedures. H. pylori is a bacterium that infects the stomach and is responsible for causing various gastrointestinal disorders, including gastritis, peptic ulcers, and even stomach cancer. Non-invasive testing methods provide a convenient and reliable means of detecting H. pylori infection, allowing for early diagnosis and appropriate treatment.
Meaning
Non-invasive testing for H. pylori refers to diagnostic methods that do not require invasive procedures such as endoscopy or biopsy. These tests typically involve the analysis of patient samples, such as breath, stool, or blood, to detect the presence of H. pylori bacteria or its associated antibodies. Non-invasive testing methods offer several advantages over invasive procedures, including lower costs, reduced patient discomfort, and increased accessibility, making them a preferred choice for diagnosing H. pylori infections.
Executive Summary
The Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing market is experiencing significant growth due to the rising incidence of H. pylori infections worldwide. The market is driven by the increasing awareness about the health risks associated with H. pylori, the availability of advanced non-invasive testing methods, and the growing demand for accurate and timely diagnosis. However, market growth is also influenced by certain restraints, such as the lack of reimbursement policies and the high cost of non-invasive testing procedures. Nevertheless, market opportunities are emerging with the development of innovative testing technologies and the expansion of healthcare infrastructure in developing regions.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Several factors are driving the growth of the global Helicobacter Pylori non-invasive testing market:
Market Restraints
Despite its growth prospects, the H. Pylori non-invasive testing market faces several challenges:
Market Opportunities
The H. Pylori non-invasive testing market presents several opportunities for growth:
Market Dynamics
The H. Pylori non-invasive testing market is influenced by a variety of dynamics, including:
Regional Analysis
The H. Pylori non-invasive testing market is segmented by regions, with varying adoption rates based on healthcare infrastructure and regional healthcare needs:
Competitive Landscape
Leading Companies in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
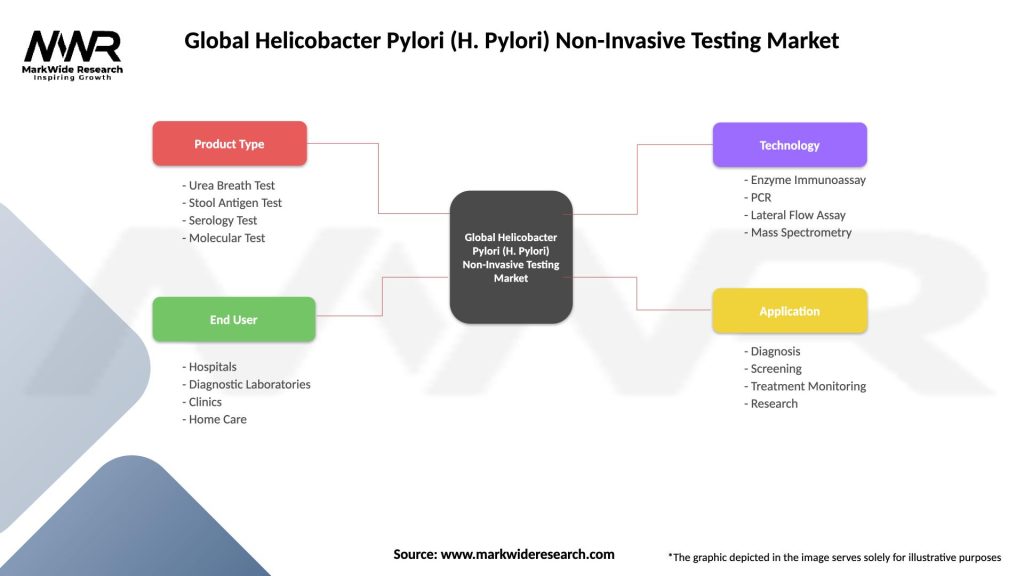
Segmentation
The Global H. Pylori Non-Invasive Testing Market is segmented based on various factors:
Category-wise Insights
Each test category offers distinct benefits and applications:
Key Benefits for Industry Participants and Stakeholders
The market provides key benefits for industry stakeholders:
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Key trends driving the market:
Covid-19 Impact
The Covid-19 pandemic has emphasized the need for non-invasive diagnostic testing, driving further interest in alternatives like H. Pylori breath tests that do not require physical contact or hospitalization.
Key Industry Developments
Recent industry developments include:
Analyst Suggestions
Future Outlook
The Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market is expected to continue growing as demand for comfortable, accurate, and cost-effective diagnostic solutions rises across the globe. Advances in diagnostic technology, coupled with expanding healthcare access, will drive further market expansion.
Conclusion
In conclusion, the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market is evolving rapidly with advancements in testing technology and increasing patient demand for non-invasive diagnostics. The market presents significant opportunities for growth, innovation, and market expansion, particularly in emerging regions where healthcare access is improving.
What is Helicobacter Pylori (H. Pylori) Non-Invasive Testing?
Helicobacter Pylori (H. Pylori) Non-Invasive Testing refers to diagnostic methods used to detect the presence of H. Pylori bacteria in the stomach without the need for invasive procedures. Common non-invasive tests include breath tests, stool antigen tests, and serological tests.
What are the key players in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market?
Key players in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market include Abbott Laboratories, Thermo Fisher Scientific, and F. Hoffmann-La Roche, among others.
What are the growth factors driving the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market?
The growth of the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market is driven by increasing prevalence of H. Pylori infections, rising awareness about gastrointestinal diseases, and advancements in diagnostic technologies.
What challenges does the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market face?
Challenges in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market include the accuracy of tests, variations in testing protocols, and the potential for misdiagnosis, which can affect treatment outcomes.
What opportunities exist in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market?
Opportunities in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market include the development of novel testing methods, increasing demand for point-of-care testing, and expanding healthcare access in emerging markets.
What trends are shaping the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market?
Trends in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market include the integration of digital health technologies, the rise of home testing kits, and a focus on personalized medicine approaches.
Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market
| Segmentation Details | Description |
|---|---|
| Product Type | Urea Breath Test, Stool Antigen Test, Serology Test, Molecular Test |
| End User | Hospitals, Diagnostic Laboratories, Clinics, Home Care |
| Technology | Enzyme Immunoassay, PCR, Lateral Flow Assay, Mass Spectrometry |
| Application | Diagnosis, Screening, Treatment Monitoring, Research |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at