444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Global Fecal Occult Blood Tests (FOBT) market refers to the worldwide market for tests that detect the presence of blood in the stool, which could indicate the presence of colorectal cancer or other gastrointestinal disorders. FOBTs are non-invasive and commonly used as a screening tool for early detection of colorectal cancer. These tests are cost-effective and easily accessible, making them a preferred choice for population-based screening programs.
Meaning
Fecal Occult Blood Tests, also known as stool blood tests, are diagnostic tests used to identify the presence of hidden or occult blood in the stool. These tests can detect tiny amounts of blood that may not be visible to the naked eye. The presence of blood in the stool may be an indication of various conditions, including colorectal cancer, polyps, ulcers, hemorrhoids, and other gastrointestinal disorders. By detecting blood in the stool, FOBTs can help in the early detection and prevention of colorectal cancer.
Executive Summary
The Global Fecal Occult Blood Tests market is experiencing significant growth due to the increasing prevalence of colorectal cancer and the growing emphasis on early diagnosis and prevention. FOBTs offer a convenient and cost-effective method for screening individuals at risk of colorectal cancer, leading to improved patient outcomes. The market is witnessing technological advancements, such as the introduction of advanced immunochemical-based FOBTs, which offer higher sensitivity and specificity compared to traditional guaiac-based tests. These advancements are driving market growth and expanding the application of FOBTs in various healthcare settings.
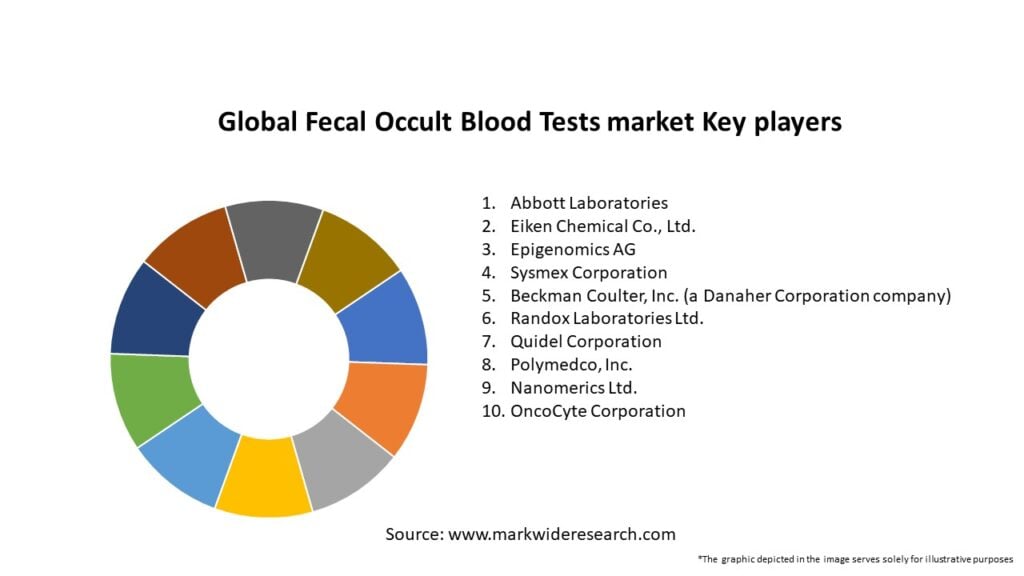
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
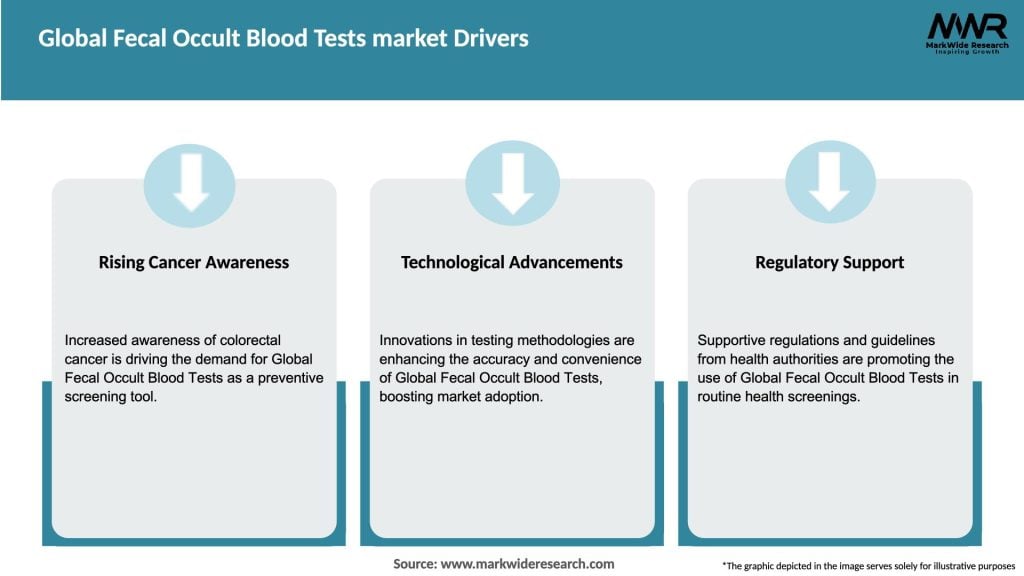
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
Regional Analysis
The FOBT market is geographically diverse, with significant demand in North America, Europe, and Asia-Pacific:
Competitive Landscape
Leading Companies in the Gobal Fecal Occult Blood Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
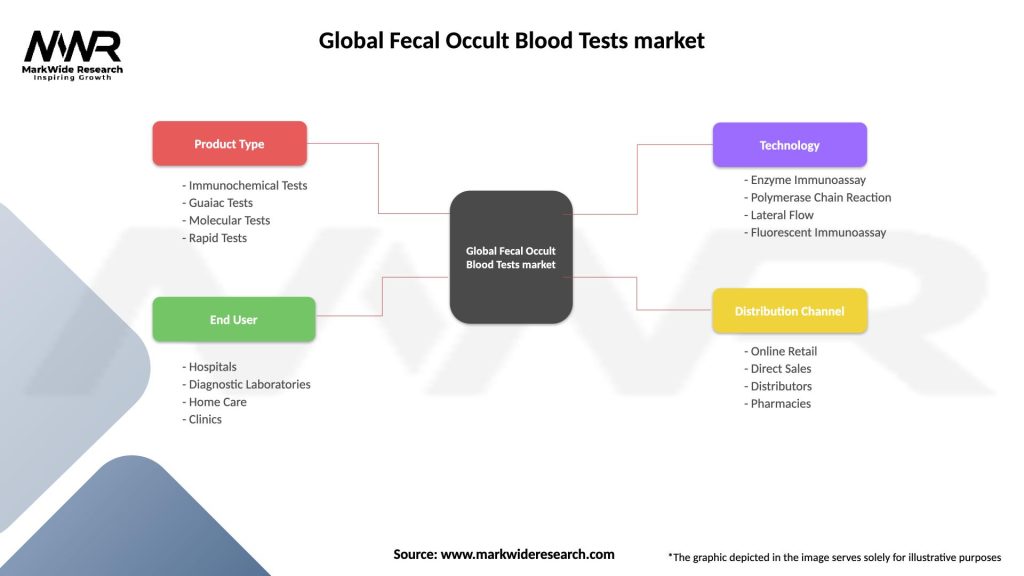
Segmentation
The Global Fecal Occult Blood Tests market can be segmented based on test type, end-user, and region.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The Global Fecal Occult Blood Tests market offers several benefits for industry participants and stakeholders, including:
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the Global Fecal Occult Blood Tests market. The outbreak disrupted healthcare services globally, diverting resources and attention towards managing the pandemic. Several routine screening programs, including colorectal cancer screening, were temporarily halted or delayed, leading to a decline in the demand for FOBTs.
However, as the situation gradually improves, healthcare systems are adapting to the new normal. The importance of early detection and prevention of colorectal cancer remains a priority, and screening programs are being resumed or redesigned to ensure patient safety. FOBTs offer a convenient and cost-effective option for screening, especially in the post-pandemic scenario where healthcare resources may still be constrained.
The Covid-19 pandemic has also accelerated the adoption of digital technologies in healthcare, including remote patient monitoring and telehealth. These technologies can be leveraged to enhance the accessibility and efficiency of FOBT screening programs, enabling patients to undergo testing from the comfort of their homes.
Key Industry Developments
Analyst Suggestions
Focus on Technological Advancements: Companies should invest in improving the sensitivity and accuracy of FOBTs, particularly through immunochemical tests (iFOBT), to reduce false positives and negatives.
Expand Home-Based Testing Options: With the growing demand for convenience and privacy, manufacturers should develop more accessible and user-friendly at-home FOBT kits, targeting both developed and emerging markets.
Increase Awareness in Emerging Markets: Efforts should be made to educate healthcare providers and the public about the importance of colorectal cancer screening, particularly in regions with low adoption rates.
Leverage Government Screening Programs: Companies should partner with governments to integrate FOBTs into national health programs, offering a broader reach and more consistent market growth.
Explore Integration with Other Diagnostics: To enhance screening capabilities, FOBTs could be integrated with genetic tests or imaging techniques to provide more comprehensive and accurate results.
Future Outlook
The global fecal occult blood tests market is poised for significant growth in the coming years. With an increasing prevalence of colorectal cancer and the growing adoption of screening programs, the demand for fecal occult blood tests is expected to surge. Furthermore, advancements in technology and the development of non-invasive testing methods are likely to drive market expansion. One of the key factors contributing to the positive future outlook of the market is the rising awareness about the importance of early detection of colorectal cancer. Governments and healthcare organizations across the globe are actively promoting screening programs to detect and prevent colorectal cancer at an early stage. This is expected to result in a higher uptake of fecal occult blood tests, as they are considered one of the most effective and cost-efficient screening methods.
Another significant driver for the market is the continuous development of innovative testing methods. Traditional fecal occult blood tests involve the detection of blood in stool samples, which can be inconvenient and unpleasant for patients. However, new technologies such as immunochemical tests and DNA-based tests are emerging as more accurate and user-friendly alternatives. These advancements are expected to attract more patients and healthcare providers to adopt fecal occult blood tests as part of routine screening protocols.
In addition to colorectal cancer screening, the application of fecal occult blood tests is expanding to other medical conditions. The tests are being increasingly used to detect gastrointestinal bleeding, inflammatory bowel diseases, and other gastrointestinal disorders. This widening scope of applications is anticipated to further fuel the market growth in the coming years. Moreover, the market is witnessing a shift toward decentralized testing facilities. Point-of-care testing (POCT) and home-based testing kits are gaining popularity due to their convenience and ease of use. Patients can now perform the tests in the comfort of their own homes, eliminating the need for clinic visits. This trend is expected to boost the demand for fecal occult blood tests and reshape the market landscape.
Conclusion
In conclusion, the global fecal occult blood tests market is projected to experience substantial growth in the foreseeable future. Factors such as increased awareness about colorectal cancer screening, technological advancements in testing methods, expanding applications of fecal occult blood tests, and the shift toward decentralized testing facilities are all contributing to the market’s positive outlook. With the growing burden of colorectal cancer worldwide, early detection and prevention have become paramount. Fecal occult blood tests provide a reliable and cost-effective screening option, making them a crucial tool in the fight against colorectal cancer. As healthcare systems and governments continue to prioritize screening programs, the demand for these tests is expected to rise.
Additionally, the development of non-invasive testing methods and the expansion of applications beyond colorectal cancer screening further enhance the market’s potential. The introduction of user-friendly and convenient testing options, such as point-of-care testing and home-based kits, is expected to drive patient compliance and increase the overall adoption of fecal occult blood tests. In summary, the future of the global fecal occult blood tests market looks promising. As advancements in technology and healthcare practices continue, the market is poised for significant growth, ultimately leading to improved early detection, better patient outcomes, and a reduction in the burden of colorectal cancer worldwide.
What is Fecal Occult Blood Tests?
Fecal Occult Blood Tests (FOBT) are medical tests used to detect hidden blood in stool samples, which can be an early indicator of gastrointestinal issues, including colorectal cancer. These tests are crucial for screening and diagnosing various digestive disorders.
What are the key players in the Global Fecal Occult Blood Tests market?
Key players in the Global Fecal Occult Blood Tests market include companies like Abbott Laboratories, Siemens Healthineers, and Quidel Corporation, which are known for their innovative diagnostic solutions and extensive product portfolios in the healthcare sector, among others.
What are the growth factors driving the Global Fecal Occult Blood Tests market?
The Global Fecal Occult Blood Tests market is driven by increasing awareness of colorectal cancer screening, advancements in diagnostic technologies, and a growing aging population that is more susceptible to gastrointestinal diseases.
What challenges does the Global Fecal Occult Blood Tests market face?
Challenges in the Global Fecal Occult Blood Tests market include the potential for false positives and negatives, patient reluctance to undergo testing, and the need for improved accuracy and reliability in test results.
What opportunities exist in the Global Fecal Occult Blood Tests market?
Opportunities in the Global Fecal Occult Blood Tests market include the development of more sensitive and specific testing methods, integration of digital health technologies for remote monitoring, and expanding awareness campaigns to encourage regular screening.
What trends are shaping the Global Fecal Occult Blood Tests market?
Trends in the Global Fecal Occult Blood Tests market include the shift towards non-invasive testing methods, the use of artificial intelligence for data analysis, and increasing collaborations between diagnostic companies and healthcare providers to enhance screening programs.
Global Fecal Occult Blood Tests market
| Segmentation Details | Description |
|---|---|
| Product Type | Immunochemical Tests, Guaiac Tests, Molecular Tests, Rapid Tests |
| End User | Hospitals, Diagnostic Laboratories, Home Care, Clinics |
| Technology | Enzyme Immunoassay, Polymerase Chain Reaction, Lateral Flow, Fluorescent Immunoassay |
| Distribution Channel | Online Retail, Direct Sales, Distributors, Pharmacies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Gobal Fecal Occult Blood Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at